Overview
Chile Contract Research Organizations (CROs) play a vital role in the healthcare sector by providing specialized services that streamline the development of medical devices and pharmaceuticals, ensuring compliance with regulatory standards and enhancing research efficiency. The article emphasizes that these organizations, such as bioaccess®, not only tackle challenges like regulatory hurdles and patient recruitment but also drive innovation and improve patient outcomes through their comprehensive management services and strategic collaborations, highlighting their growing importance in the global Medtech landscape.
Introduction
In the evolving landscape of healthcare, Contract Research Organizations (CROs) in Chile have emerged as pivotal players in facilitating clinical trials and advancing medical device development. These organizations, such as bioaccess®, provide a comprehensive suite of services that address the multifaceted challenges faced by pharmaceutical and biotechnology firms. From navigating complex regulatory environments to enhancing patient access to innovative therapies, CROs are instrumental in streamlining processes that ultimately lead to improved healthcare outcomes.
As the demand for specialized CRO services continues to grow, understanding their role and the services they offer becomes essential for stakeholders aiming to harness the potential of clinical research in Chile. This article delves into the critical functions of CROs, the challenges they encounter, and the future trends shaping their operations within the healthcare sector.
Defining Contract Research Organizations in Chile
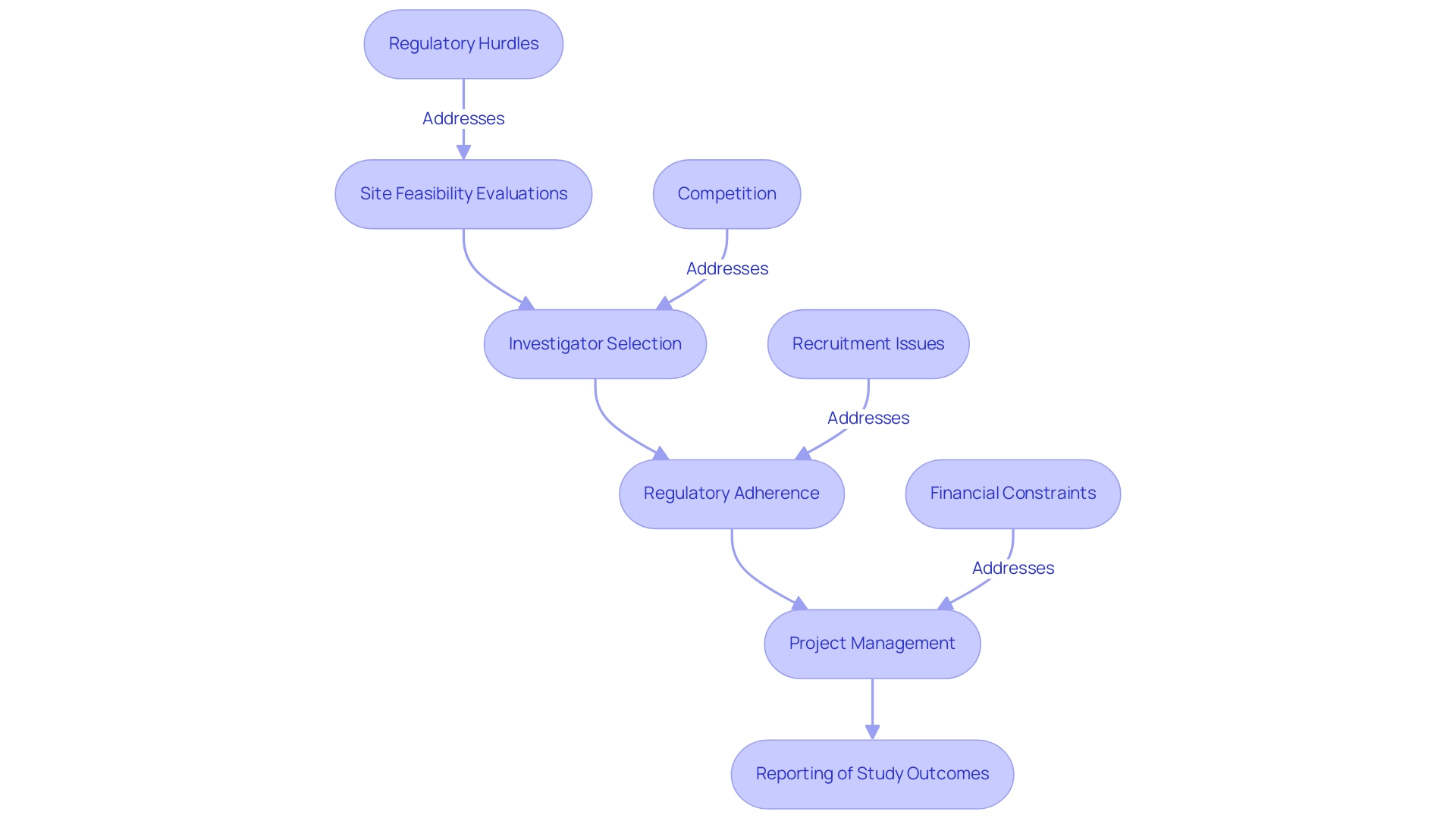
Chile Contract Research Organizations, such as bioaccess®, play a crucial role in promoting the development of medical devices by offering outsourced services tailored for the pharmaceutical, biotechnology, and medical device industries. These organizations offer a thorough procedure that encompasses:
- Site feasibility evaluations
- Investigator selection
- Regulatory adherence
- Project management
- Reporting of study outcomes, including oversight of ongoing studies
As essential partners, Chile Contract Research Organizations like bioaccess® simplify study management, tackling the challenges encountered by medical device startups, such as:
- Regulatory hurdles
- Competition
- Recruitment issues
- Financial constraints
With a commitment to high-quality research, bioaccess® ensures compliance with both international standards and local regulations, thereby enhancing the integrity of trials. The organization's expertise enables swift regulatory approval, research site activation, and participant recruitment, which are essential for Medtech startups pursuing accelerated results. Moreover, the rigorous oversight by the local Ministry of Health, Food and Drug Administration, and European Medicines Agency underscores the quality of medical research conducted by the Chile Contract Research Organization.
As the Chile Contract Research Organization sector is projected to grow at a CAGR of 9.6%, the significance of these organizations as strategic allies in healthcare innovation is increasingly acknowledged. By staying informed with market intelligence, firms can anticipate industry trends and capitalize on emerging opportunities, positioning them favorably in the competitive landscape. To learn more about how bioaccess® can assist in advancing your medical device project, BOOK A MEETING today.
The Importance of CROs in Chile's Healthcare Sector
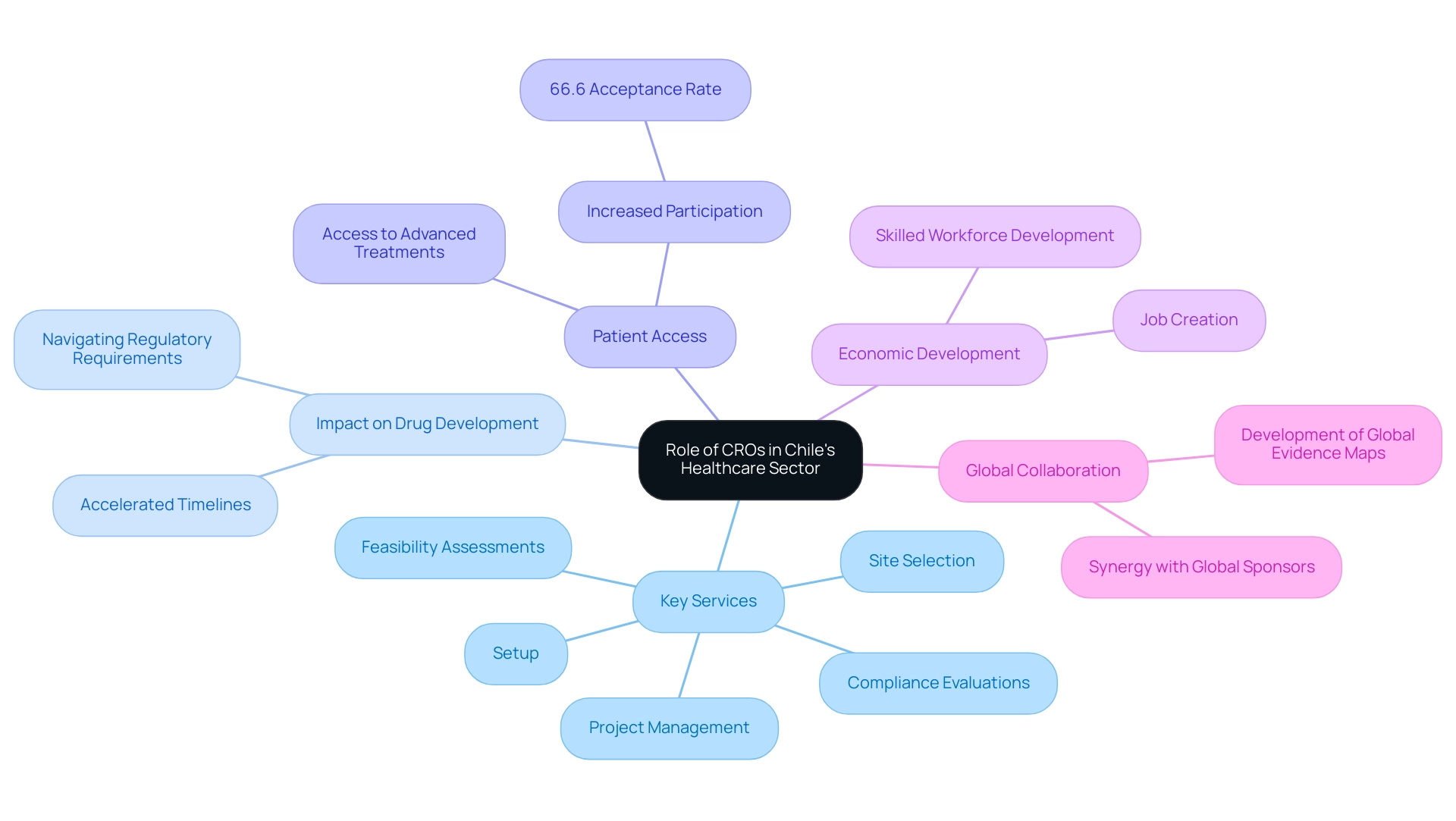
The Chile Contract Research Organization plays a crucial role in Chile's healthcare sector by streamlining the implementation of research studies, which are essential for the introduction of innovative therapies. Their extensive knowledge includes thorough study management services, such as:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Project management
This proficiency allows pharmaceutical companies to deftly navigate complex regulatory requirements, significantly accelerating drug development timelines.
Significantly, recent information shows that 66.6% of participants reported no resistance from patients when approached to enroll in research studies, indicating a growing acceptance and accessibility of these investigations. Additionally, contract research organizations improve patient access to research studies, allowing individuals to obtain advanced treatments frequently inaccessible through traditional healthcare routes. The ongoing growth of Chile Contract Research Organization corresponds with a worldwide pattern in medical research, encouraging cooperation between local companies and global sponsors.
This synergy not only stimulates innovation but also enhances patient outcomes across the region. As noted by Manuel A. Espinoza, 'Chile has made significant progress in recent decades in implementing policies to improve the efficiency of its health system with an impact on population health.' This demonstrates the essential role of Chile Contract Research Organization in propelling progress in medical research and healthcare provision.
Furthermore, the creation of global evidence maps for prioritized conditions, supported by high or moderate certainty international research categorized according to the GRADE approach, highlights the role of Chile Contract Research Organization in improving the quality and reliability of evidence in Chile. Additionally, the case study on health benefit plans in Chile highlights how the Chile Contract Research Organization has played a role in improving access to healthcare services and providing financial protection for the population. Moreover, Chile Contract Research Organization aids in job creation and economic development by cultivating a skilled workforce in research, thus enhancing the local economy.
This emphasizes the integral role of clinical research organizations in shaping the healthcare landscape and driving international collaboration in Medtech.
Key Services Offered by CROs in Chile
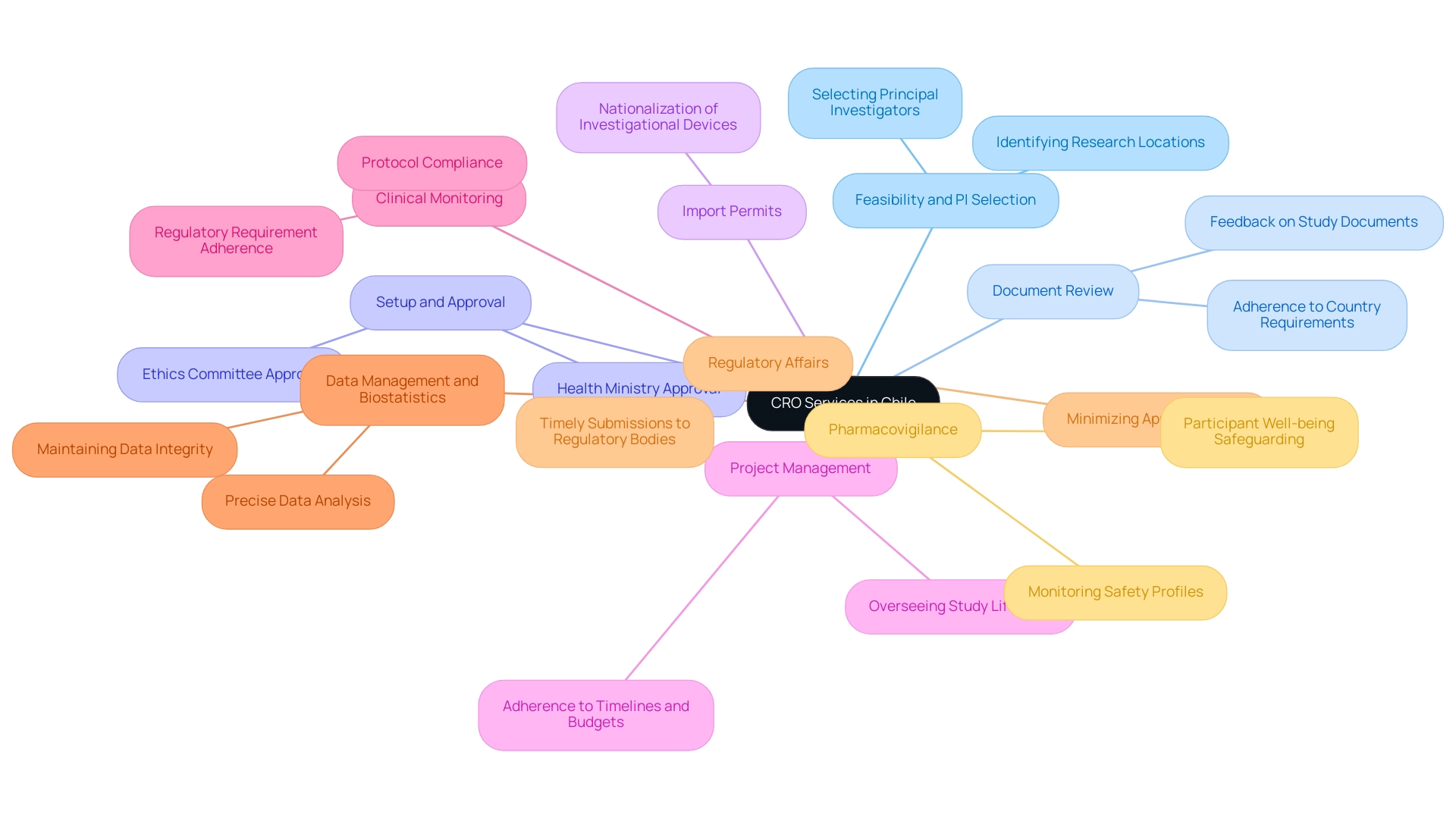
In Chile, the Chile Contract Research Organization plays a crucial part in the successful implementation of clinical studies by providing a complete range of offerings vital for upholding study integrity and efficiency. Our operational capabilities encompass:
- Feasibility and selection of research locations and principal investigators (PIs)
- Review and feedback on study documents to adhere to country requirements
- Setup, initiation, and approval from ethics committees and health ministries
- Import permits and the nationalization of investigational devices
Among these offerings, project management stands out as a foundational element, overseeing the entire study lifecycle and ensuring strict adherence to timelines and budgets.
This critical function not only facilitates smooth operations but also enhances the overall quality of research outcomes. Clinical monitoring is another essential service, ensuring that studies are conducted in accordance with established protocols and regulatory requirements. Meanwhile, data management and biostatistics concentrate on maintaining the integrity and precise analysis of study data, essential for deriving valid conclusions.
Moreover, Regulatory Affairs specialists within CROs ensure that all necessary submissions to regulatory bodies are precise and timely, minimizing potential delays in the approval process. Pharmacovigilance is equally essential, as it involves continuous monitoring of the safety profile of study medications throughout the research phase, thus safeguarding participant well-being. The collaborative efforts of these specialists reflect a national strategy aimed at reducing health inequities, ultimately improving access and care quality—a goal that resonates strongly within the context of Chile's ongoing public health initiatives.
As highlighted by the local Ministry of Health, Food and Drug Administration, and European Medicines Agency, the rigorous inspections validate the high quality of clinical trials conducted in Chile, further underscoring the importance of effective project management and comprehensive reporting in clinical research. With around 50 million Americans impacted by neurological conditions, the assistance offered by clinical research organizations becomes increasingly important in tackling major health challenges. Additionally, the Cervical Cancer Prevention and Control Program in Chile serves as a pertinent case study, showcasing the impact of the Chile Contract Research Organization's services and public health initiatives in reducing cancer incidence rates.
Challenges Faced by CROs in Chile
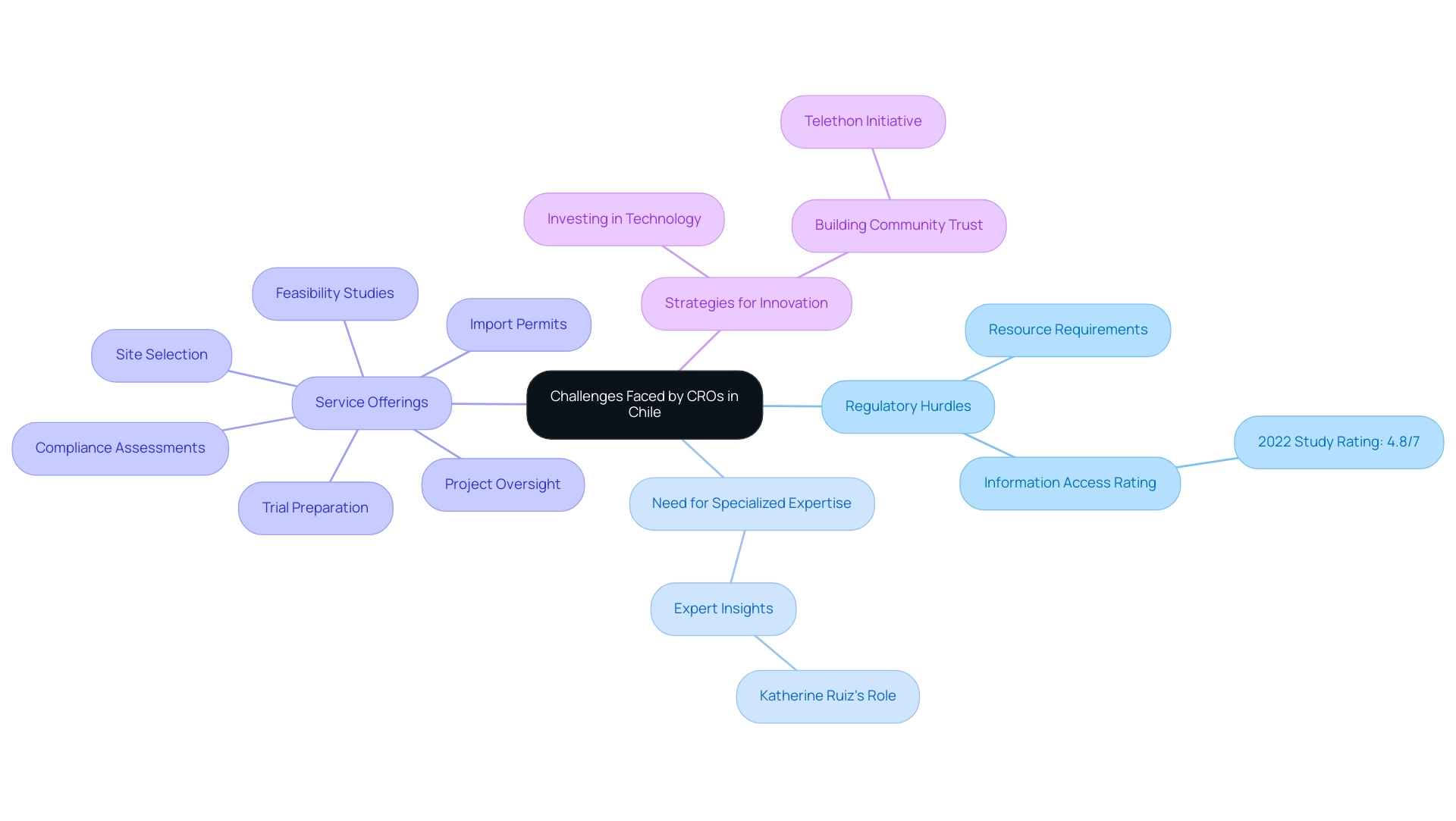
The Chile Contract Research Organization faces a complicated environment of obstacles that greatly affect their operational effectiveness and the success of research studies. Regulatory hurdles are particularly formidable, with a 2022 study by the Council of Transparency rating the information access system at 4.8 on a scale of 1 to 7. This underscores the necessity for a Chile Contract Research Organization to adeptly navigate the evolving regulatory framework, which requires substantial resources and specialized expertise.
Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, exemplifies the high level of proficiency needed in this field to drive compliance and innovation.
The Chile Contract Research Organization provides a variety of thorough clinical trial management solutions, encompassing:
- Feasibility studies
- Site selection
- Compliance assessments
- Trial preparation
- Import permits
- Project oversight
Experts highlight that distinguishing offerings is crucial in today's competitive environment. As one industry leader articulated, "Chile Contract Research Organizations must not only adhere to regulations but also innovate to cater to their clients' unique needs."
This insight highlights the critical importance of delivering exceptional service quality and innovative solutions. To maintain a competitive edge, clinical research organizations must invest in advanced technologies and comprehensive training for their teams, which, while resource-intensive, is vital for relevance in the dynamic healthcare sector.
Moreover, a case study focusing on social capital and trust in Chile illustrates the value of collective action in addressing these challenges. Despite the low levels of interpersonal trust, initiatives like the Telethon demonstrate that Chile Contract Research Organizations can foster solidarity and collaboration, enhancing their operational capabilities. By leveraging community initiatives, Chile Contract Research Organizations can build trust and improve their service delivery.
Addressing these challenges is vital for chief risk officers in Chile, particularly as forecasts suggest a return to positive growth rates in 2024, anticipated to reach 2.5% of GDP. By framing these challenges as opportunities, the Chile Contract Research Organization can adapt and thrive in an increasingly intricate market, ultimately driving global health improvement through international collaboration and innovation in Medtech.
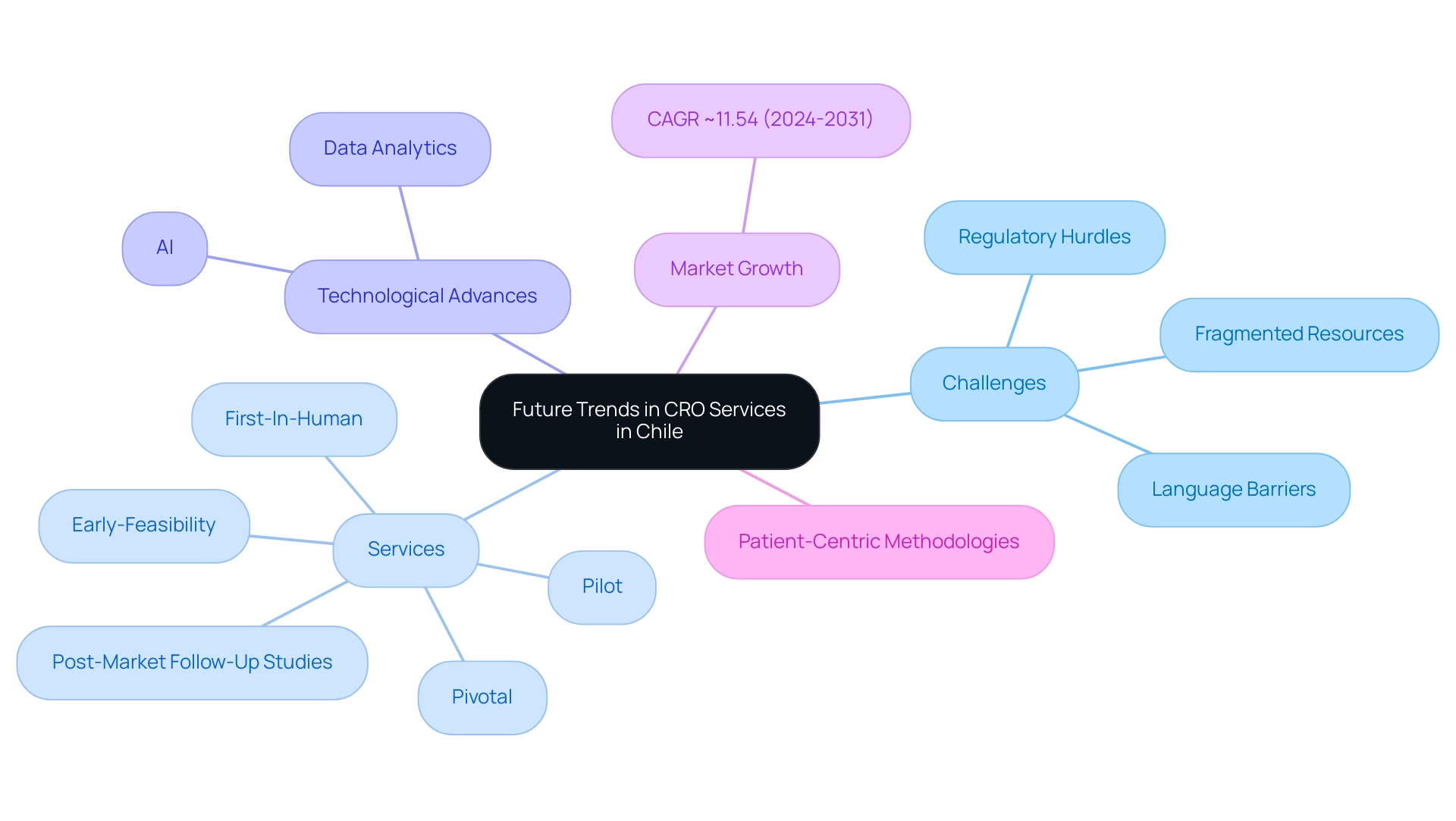
Future Trends in CRO Services in Chile
The future landscape of the Chile Contract Research Organization (CRO) sector is poised for significant transformation due to several pivotal trends, particularly in the context of Medtech innovations. Challenges such as regulatory hurdles, language barriers, and fragmented resources in Latin America have underscored the need for specialized expertise and collaboration, particularly between organizations like Greenlight Guru and bioaccess™. By utilizing over 20 years of experience, bioaccess® provides comprehensive solutions for:
- Early-Feasibility
- First-In-Human
- Pilot
- Pivotal
- Post-Market Follow-Up Studies
Advanced technologies like artificial intelligence and data analytics are poised to improve trial efficiency, streamlining processes such as patient recruitment and data management, ultimately resulting in shorter trial durations and decreased costs. The CRO market is expected to grow at a CAGR of ~11.54% from 2024 to 2031, reflecting the increasing demand for these specialized services. This growth underscores the urgency of adopting a solution-driven approach to bridge the gap between innovation and execution in the clinical research landscape.
Furthermore, a notable shift towards patient-centric methodologies emphasizes improving participant experiences, which is crucial for fostering engagement throughout the trial process. As globalization continues to shape the healthcare environment, Chile Contract Research Organizations are likely to forge strategic collaborations with international partners, thereby broadening their operational capabilities and market reach. These developments illustrate the ongoing innovation within the CRO sector, enhancing service offerings and operational capabilities; adapting to these trends will be essential for CROs striving to maintain a competitive edge in this dynamic sector.
Conclusion
The role of Contract Research Organizations (CROs) in Chile's healthcare landscape is becoming increasingly vital as they facilitate clinical trials and the development of medical devices. By offering a comprehensive suite of services, including:
- Regulatory compliance
- Project management
- Patient recruitment
CROs like bioaccess® are essential partners for pharmaceutical and biotechnology firms aiming to navigate complex regulatory environments efficiently. The growth projection of the CRO market emphasizes their importance in driving healthcare innovation, enhancing patient access to cutting-edge treatments, and ultimately improving health outcomes across the region.
Despite facing challenges such as regulatory hurdles and the need for innovative solutions, CROs are adapting by investing in advanced technologies and fostering collaboration. This proactive approach not only addresses the unique needs of clients but also positions CROs as key players in the evolving landscape of clinical research. The emphasis on patient-centric methodologies and strategic partnerships further underscores the commitment of CROs to enhance the overall quality of healthcare delivery in Chile.
As the demand for specialized CRO services continues to grow, stakeholders must recognize the integral role these organizations play in shaping the future of clinical research. By leveraging their expertise and adapting to emerging trends, CROs will remain pivotal in advancing healthcare solutions and driving international collaboration in the Medtech sector. Understanding and supporting the work of CROs is essential for fostering an environment conducive to innovation and improving patient outcomes in Chile and beyond.
Frequently Asked Questions
What role do Chile Contract Research Organizations (CROs) play in the development of medical devices?
Chile CROs, such as bioaccess®, provide outsourced services that facilitate the development of medical devices by conducting site feasibility evaluations, investigator selection, regulatory adherence, project management, and reporting of study outcomes.
What challenges do medical device startups face that CROs help to address?
CROs assist medical device startups in overcoming challenges such as regulatory hurdles, competition, recruitment issues, and financial constraints.
How do CROs ensure compliance and quality in research studies?
Organizations like bioaccess® ensure compliance with international standards and local regulations through rigorous oversight by entities such as the local Ministry of Health, Food and Drug Administration, and European Medicines Agency, enhancing the integrity of clinical trials.
What is the projected growth rate for the Chile CRO sector?
The Chile CRO sector is projected to grow at a compound annual growth rate (CAGR) of 9.6%.
How do CROs improve patient access to research studies?
CROs improve patient access by facilitating enrollment in research studies, which often provide advanced treatments that are not available through traditional healthcare avenues.
What recent findings indicate the acceptance of research studies among patients in Chile?
Recent data shows that 66.6% of participants reported no resistance when approached to enroll in research studies, reflecting a growing acceptance of these investigations.
What impact do CROs have on the healthcare system in Chile?
CROs play a crucial role in improving the efficiency of the health system, enhancing population health, and stimulating innovation, which leads to better patient outcomes.
How do CROs contribute to job creation and economic development in Chile?
CROs cultivate a skilled workforce in research, contributing to job creation and enhancing the local economy.




