Overview
Choosing the right Medical Device CRO in Chile involves evaluating key factors such as regulatory compliance, experience with medical devices, local knowledge, and project management capabilities. The article emphasizes that a thorough assessment of these criteria, along with the CRO's track record and communication skills, is essential for ensuring successful clinical trials, supported by examples of effective collaborations and improvements in recruitment timelines.
Introduction
In the dynamic landscape of medical device trials, the selection of a Contract Research Organization (CRO) plays a pivotal role in ensuring successful outcomes. As the demand for innovative healthcare solutions grows, particularly in regions like Chile, understanding the nuances of choosing the right CRO becomes essential. This article delves into critical considerations for selecting a CRO, the advantages of conducting trials in Chile, and the importance of evaluating a CRO's:
- Track record
- Pricing structure
- Communication capabilities
By navigating these factors, sponsors can enhance their chances of success and contribute to the advancement of medical technology in a rapidly evolving market.
Key Considerations for Selecting a Medical Device CRO in Chile
When selecting a Medical Device CRO in Chile, several critical factors must be considered to ensure the success of clinical trials:
- Regulatory Compliance: A strong grasp of Chilean regulations is essential. The CRO should be well-versed in the requirements set forth by the Chilean Ministry of Health and applicable international guidelines, ensuring efficient navigation through the regulatory landscape.
- Experience with Medical Devices: It is essential to select CROs that focus on medical device studies and possess a demonstrated history of successful research. For instance, bioaccess® has over 20 years of experience in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, which can significantly influence study outcomes.
- Local Knowledge and Network: A CRO with established local connections can enhance interactions with regulatory bodies, ethics committees, and other stakeholders. This local insight can streamline processes and facilitate quicker approvals, as evidenced by recent partnerships like GlobalCare Clinical Studies with bioaccess™, which led to a 50% decrease in recruitment time.
- Quality of Staff: Assessing the qualifications and expertise of the CRO's team is vital. A knowledgeable and experienced team ensures that research studies are conducted effectively and comply with the highest standards.
- Project Management Capabilities: Evaluate the CRO's project management skills, particularly their ability to manage timelines, budgets, and resources. Efficient project management is essential for maintaining studies on schedule and within scope; bioaccess® demonstrates robust project management in the clinical study services, guaranteeing that all stages of the study are carefully planned and executed.
- Technology and Data Management: Consider the technological platforms utilized by the CRO for data collection, management, and analysis. Advanced technology enhances data accuracy and improves overall efficiency, crucial for successful results.
- Communication and Collaboration: The CRO should exhibit strong communication skills and a collaborative approach to working with sponsors and stakeholders. A positive working relationship throughout the process fosters better outcomes and reduces misunderstandings. Julio G. Martinez-Clark observes that "patient recruitment occurs ten times quicker than Western markets," highlighting the benefits of conducting studies in this region.
- Reporting and Transparency: It is essential for the CRO to provide regular reporting on study status, including inventory management and updates on serious and non-serious adverse events. bioaccess® ensures transparency by maintaining open lines of communication with all stakeholders, providing detailed reports that comply with regulatory requirements.
By meticulously evaluating these factors, you can make a well-informed decision in selecting a Medical Device CRO in Chile, which is pivotal in paving the way for successful clinical studies. Historical data from 2019 to 2024 suggests that the effectiveness of CROs in Chile has been steadily improving, particularly in areas such as feasibility evaluations, site selection, compliance assessments, and project management. A recent client stated, "Working with bioaccess® not only streamlined our processes but also significantly improved our recruitment timelines, leading to a successful study completion ahead of schedule.
The Advantages of Conducting Medical Device Trials in Chile
Conducting medical device trials in Chile presents numerous advantages that can significantly enhance the chances of success for sponsors, particularly in the context of the evolving Medtech landscape in Latin America, especially when contrasted with the challenges faced by U.S. Medtech companies, such as regulatory hurdles, limited financial resources, and prolonged subject recruitment timelines:
- Positive Oversight Landscape: Chile has established a helpful framework for research, marked by clear guidelines that simplify the approval process for medical device trials. This environment fosters efficiency and encourages innovation, addressing the regulatory hurdles faced by Medtech companies.
- Access to Diverse Patient Populations: The country's rich demographic diversity allows researchers to access a wide array of patient populations, which is crucial for studies that require varied participant profiles. This aspect can lead to more robust and generalizable results, bridging gaps in medical research.
- Cost-Effectiveness: Conducting experiments in Chile is often more economical than in other regions. The lower operational costs coupled with competitive pricing from the Medical Device CRO Chile make it an attractive option for many sponsors, supporting the financial viability of Medtech innovations.
- High Quality of Research: Chilean institutions are acknowledged for upholding high standards in medical research. This commitment to quality enhances the credibility and reliability of results, instilling confidence in stakeholders and facilitating collaboration between companies like Greenlight Guru and bioaccess™.
- Strong Infrastructure: The nation features a well-developed healthcare system and sophisticated medical facilities, which are crucial for the efficient implementation of research studies. These resources contribute to a conducive research environment, essential for first-in-human and pivotal studies.
- Supportive Government Initiatives: The Chilean government actively promotes medical research through various initiatives, offering essential assistance to companies seeking to carry out studies in the region. This governmental backing can facilitate smoother operations and enhance collaboration, which is vital for Medical Device CRO Chile companies navigating local landscapes.
- Growing Market for Medical Devices: As one of the expanding markets for medical devices in Latin America, utilizing a Medical Device CRO Chile to conduct studies offers valuable insights and a competitive edge for companies aiming to enter or expand within this market. This growth is evident in the yearly investment in the research sector in Latin America's Andean Region, which has increased from $3-4 million to over $50 million annually.
Moreover, as Julio G. Martinez-Clark, CEO of bioaccess, points out, "This article investigates the necessity to carry out clinical studies outside the U.S. (OUS) and explores the expansion of clinical studies in Latin America." By leveraging these advantages while also addressing the challenges of professionalism, language barriers, and resource fragmentation, sponsors can enhance the success of their medical device studies and contribute to the ongoing advancement of healthcare innovations in the regions.
Evaluating the CRO's Track Record and Reputation
To effectively evaluate the track record and reputation of a Medical Device CRO Chile in the field of medical devices, consider the following steps:
-
Review Case Studies: Investigate published case studies or project summaries that showcase the engagements of Medical Device CRO Chile in medical device evaluations. For example, the partnership between Welwaze Medical Inc. and bioaccess™ for the launch of the Celbrea® medical device in Colombia illustrates a successful entry into the Latin American market, showcasing the CRO's ability to navigate intricate compliance landscapes.
Additionally, the partnership with GlobalCare Clinical Trials, which achieved over a 50% reduction in recruitment time and 95% retention rates, further highlights the CRO's effectiveness in enhancing trial efficiency.
-
Check References: Request references from the CRO's previous clients. Engaging directly with past clients offers invaluable perspectives on their experiences, quality of service, and the CRO's overall performance.
-
Examine Compliance History: Scrutinize the CRO's compliance history for any past failures or issues. A clear compliance history acts as a strong sign of the Medical Device CRO Chile's dependability and ethical principles, which are crucial for upholding integrity in medical research. For example, bioaccess™ has successfully navigated regulatory challenges in its collaborations, ensuring compliance and facilitating smooth testing processes.
-
Assess Publication Record: Evaluate the CRO's involvement in published research. A robust portfolio of publications in reputable journals reflects their expertise and commitment to conducting high-quality research, which is crucial for the Medical Device CRO Chile trials.
-
Industry Reputation: Conduct thorough research on the CRO's reputation within the industry. Collect feedback from colleagues, industry forums, and professional associations to understand their position in the research community.
-
Understand Specific Services Offered: Familiarize yourself with the specific services provided by bioaccess™, such as Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Post-Market Clinical Follow-Up Studies (PMCF). Comprehending these services will provide you with insight into how bioaccess™ can effectively assist your trial needs.
By carefully evaluating these factors, you can ensure that you collaborate with a dependable and seasoned Medical Device CRO Chile, which will greatly enhance the success of your medical device trial. The clinical segment, which accounted for an impressive 85.1% of the medical device CRO Chile market share in 2023, underscores the critical role of selecting an adept organization in this evolving landscape. Recent developments, such as Charles River Laboratories' acquisition of SAMDI Tech to enhance their mass spectrometry solutions, illustrate how CROs are adapting to emerging technologies and compliance changes, further emphasizing the importance of selecting a CRO that is responsive to industry shifts.
Understanding the CRO's Pricing Structure
When assessing a Contract Research Organization's (CRO) pricing structure, several critical factors must be considered to ensure a thorough evaluation:
- Fee Structure: Begin by understanding the CRO's fee model—whether it operates on a flat fee, hourly rates, or per-patient costs. Each approach can significantly impact your budgeting and overall project expenditure.
- Inclusions and Exclusions: It's crucial to clarify what services are encompassed in the quoted price. Crucial services like feasibility studies, site selection, data management, compliance submissions, and monitoring should be clearly addressed to prevent unforeseen expenses in the future. Additionally, ensure that services like review and feedback on study documents and reporting on study status and adverse events are included, as these are vital for compliance and project oversight.
- Additional Costs: Engage in discussions about possible supplementary expenses that may arise during the process, including fees for extra services or unexpected compliance requirements. This proactive approach can enhance your cost management strategy.
- Payment Terms: Establish clear payment terms from the outset, detailing deposit requirements, payment schedules, and any penalties for late payments. This transparency helps in maintaining financial control throughout the project.
- Comparative Analysis: Conduct a comparative analysis of pricing structures across various CROs to ensure competitive rates for comparable services. However, remain cautious about prioritizing cost over quality; the CRO's expertise, particularly in regulatory matters and study management, and track record are paramount.
In the context of the Chilean market, which is projected to grow at a CAGR of 5.7% from 2024 to 2030, leveraging insights from Medical Device CRO Chile can enhance cost-effectiveness in studies. Notably, key companies in the market such as Novartis, Roche, Pfizer, Merck & Co, Johnson & Johnson, AbbVie, and Sanofi are instrumental players in shaping the competitive landscape of CRO services. Additionally, the local Ministry of Health, Food and Drug Administration, and European Medicines Agency have confirmed the high quality of Chilean studies, indicating a strong environment for CRO services.
This underscores the need for strategic evaluations of CRO pricing in relation to the quality of services offered, including compliance with country requirements. Additionally, the case study on North America's leadership in CRO services highlights the importance of a robust pharmaceutical industry and substantial investments in R&D, which are critical considerations for CRO selection. Recent developments, such as LabCorp's acquisition of the outreach laboratory business and selected operating assets of Baystate Health, further reflect current trends in the CRO market that may impact pricing structures and service offerings.
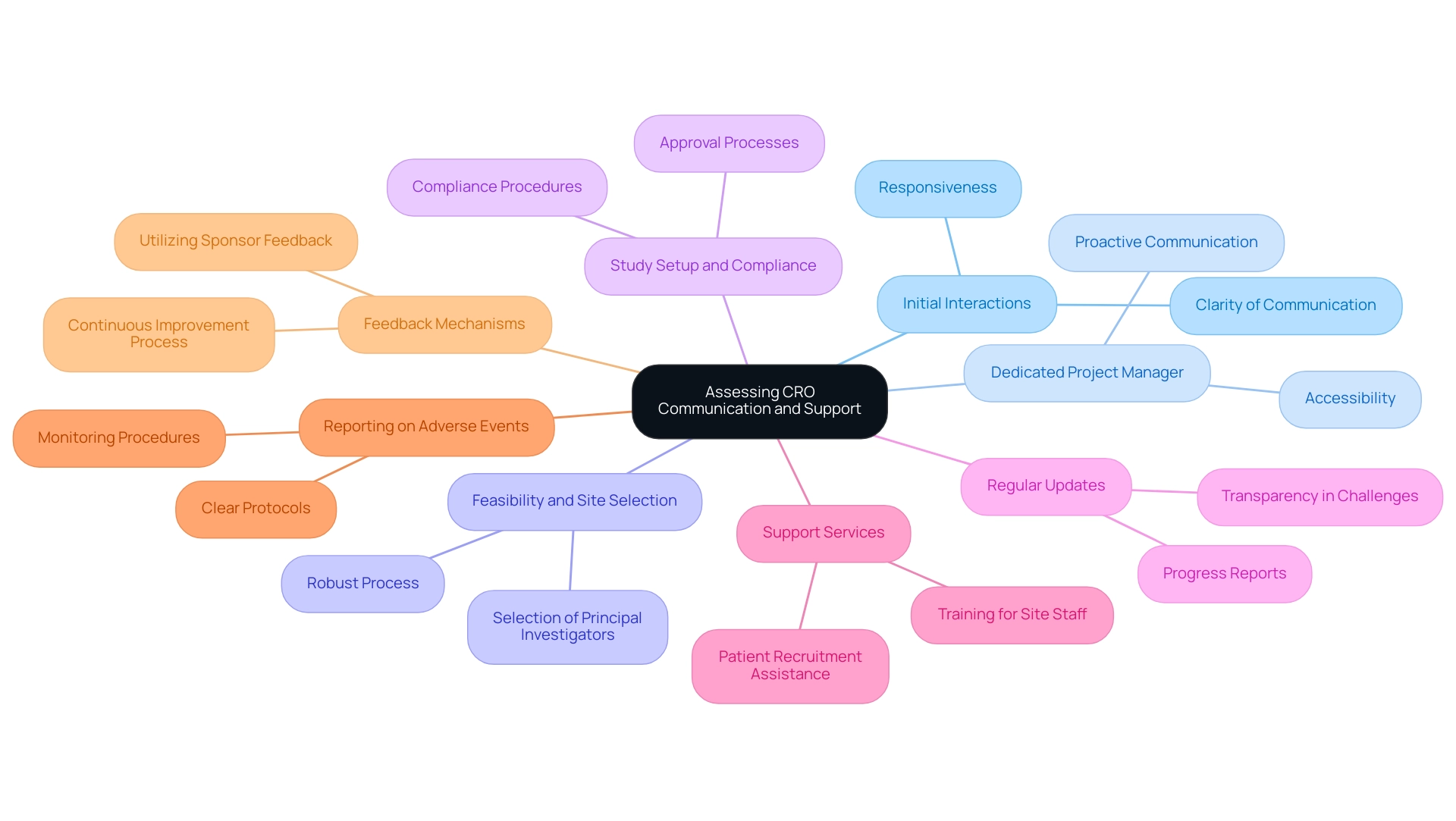
Assessing the CRO's Communication and Support
Assessing the communication and support abilities of a Medical Device CRO Chile is vital for guaranteeing the success of research studies, especially in the swiftly changing environment of medical devices. Effective communication is essential for patient engagement and retention during clinical studies, especially as more studies transition to virtual or decentralized models. Here are key aspects to consider:
- Initial Interactions: Observe the responsiveness and clarity of communication during initial discussions. This can provide valuable insight into the CRO's overall communication style and approach, reflecting their commitment to regulatory excellence.
- Dedicated Project Manager: Ensure that the CRO designates a dedicated project manager who will act as your main contact throughout the study. This individual should be easily accessible and proactive in addressing concerns, fostering a reliable line of communication that enhances project management effectiveness.
- Feasibility and Site Selection: Ensure the CRO has a robust process for feasibility studies and the selection of research sites and principal investigators (PIs). This is a critical component of successful management and can significantly impact the outcome.
- Study Setup and Compliance: Inquire about the CRO's procedures for study setup, start-up, and obtaining necessary approvals from ethics committees and health ministries. Understanding their approach to compliance is essential for regulatory excellence.
- Regular Updates: Ensure that the CRO commits to providing consistent updates on progress. This includes detailed reports on milestones, challenges encountered, and adjustments made to the study plan, which is essential for maintaining transparency and trust, especially in the context of compliance reviews and site selection.
- Support Services: Inquire about the range of support services the CRO offers, such as training for site staff, assistance with patient recruitment, and troubleshooting for study-related issues. Effective communication during these processes is crucial for improving patient involvement and retention, especially as studies become more decentralized. Regular check-ins and thorough explanations of processes can significantly improve the patient journey.
- Reporting on Adverse Events: Discuss how the CRO manages reporting on serious and non-serious adverse events, ensuring that there is a clear protocol in place for monitoring and addressing these occurrences throughout the trial.
- Feedback Mechanisms: Discuss how the CRO gathers and utilizes feedback from sponsors and stakeholders, ensuring a continuous improvement process is in place. As emphasized by industry specialist Aidan Gannon, 'We need, as an industry, to improve on that,' indicating the necessity for better communication interfaces throughout the evaluation process.
By thoroughly assessing the communication and support capabilities of a Medical Device CRO Chile through these criteria, you can foster a collaborative partnership that significantly enhances the success of your medical device evaluation. Moreover, case studies have shown that tools like text messaging can effectively keep patients informed about important updates, thereby improving their overall experience and security through features like two-factor authentication, especially in the context of virtual or decentralized trials.
Conclusion
Selecting the right Contract Research Organization (CRO) is a critical decision that can significantly influence the success of medical device trials. This article has highlighted several essential factors to consider during the selection process, including the CRO's:
- Track record
- Pricing structure
- Communication capabilities
- Specific expertise in the regulatory landscape of Chile
By carefully evaluating these elements, sponsors can ensure a more streamlined trial process, ultimately leading to better outcomes.
Conducting trials in Chile offers numerous advantages, such as:
- A favorable regulatory environment
- Access to diverse patient populations
- Cost-effectiveness
These factors contribute to an increasingly supportive landscape for Medtech innovations, making Chile an attractive location for clinical research. The growing investment in the clinical trial sector reflects the potential for successful outcomes when partnering with the right CRO.
In conclusion, a strategic approach to selecting a CRO that aligns with the specific needs of medical device trials can enhance the likelihood of success. By prioritizing a CRO's experience, communication practices, and pricing transparency, sponsors can establish partnerships that not only facilitate efficient trial execution but also contribute to the advancement of healthcare technologies in a rapidly evolving market. As the landscape of medical device trials continues to evolve, making informed decisions will be paramount to achieving desired results and fostering innovation in the industry.
Frequently Asked Questions
What factors should be considered when selecting a Medical Device CRO in Chile?
Key factors include regulatory compliance, experience with medical devices, local knowledge and network, quality of staff, project management capabilities, technology and data management, communication and collaboration, and reporting and transparency.
Why is regulatory compliance important when choosing a CRO?
A CRO must have a strong grasp of Chilean regulations and international guidelines to efficiently navigate the regulatory landscape, ensuring that clinical trials meet all necessary requirements.
How does experience with medical devices influence the selection of a CRO?
Selecting CROs that specialize in medical device studies and have a proven track record can significantly affect the success of research studies.
What role does local knowledge and network play in the effectiveness of a CRO?
A CRO with established local connections can facilitate interactions with regulatory bodies and ethics committees, streamlining processes and potentially leading to quicker approvals.
Why is the quality of staff important in a CRO?
The qualifications and expertise of the CRO's team are critical to ensuring that research studies are conducted effectively and adhere to high standards.
What should be evaluated regarding a CRO's project management capabilities?
It's essential to assess the CRO's ability to manage timelines, budgets, and resources to ensure studies remain on schedule and within scope.
How does technology and data management impact clinical trials?
Advanced technological platforms used by a CRO enhance data accuracy and improve overall efficiency, which is crucial for achieving successful results.
What is the significance of communication and collaboration in CRO operations?
Strong communication skills and a collaborative approach foster better outcomes and reduce misunderstandings between the CRO, sponsors, and stakeholders.
What is expected regarding reporting and transparency from a CRO?
A CRO should provide regular updates on study status, including inventory management and reports on adverse events, ensuring compliance with regulatory requirements.
What advantages does conducting medical device trials in Chile offer?
Advantages include a positive oversight landscape, access to diverse patient populations, cost-effectiveness, high quality of research, strong infrastructure, supportive government initiatives, and a growing market for medical devices.




