Overview
Early feasibility studies for medical devices serve as crucial preliminary assessments that evaluate the safety and practicality of innovative medical instruments before they enter full-scale clinical trials. The article highlights their importance in identifying risks, validating technology, and navigating regulatory requirements, ultimately enhancing the efficiency of medical device development and improving patient safety.
Introduction
The realm of early feasibility studies (EFS) has emerged as a critical phase in the development of innovative medical devices, particularly within the dynamic landscape of Latin America. These preliminary investigations not only assess the viability of new technologies but also play a pivotal role in ensuring patient safety and regulatory compliance before advancing to larger clinical trials.
By examining case studies and recent advancements, this article delves into the essential components of EFS, including:
- Risk assessment
- Technology validation
- Navigation of regulatory requirements
It also addresses the myriad challenges that researchers face, such as:
- Participant recruitment
- Logistical hurdles
While highlighting the innovative trends shaping the future of medical device development. As organizations strive to enhance clinical investigation strategies, understanding the intricacies of early feasibility studies becomes paramount for driving successful outcomes in healthcare.
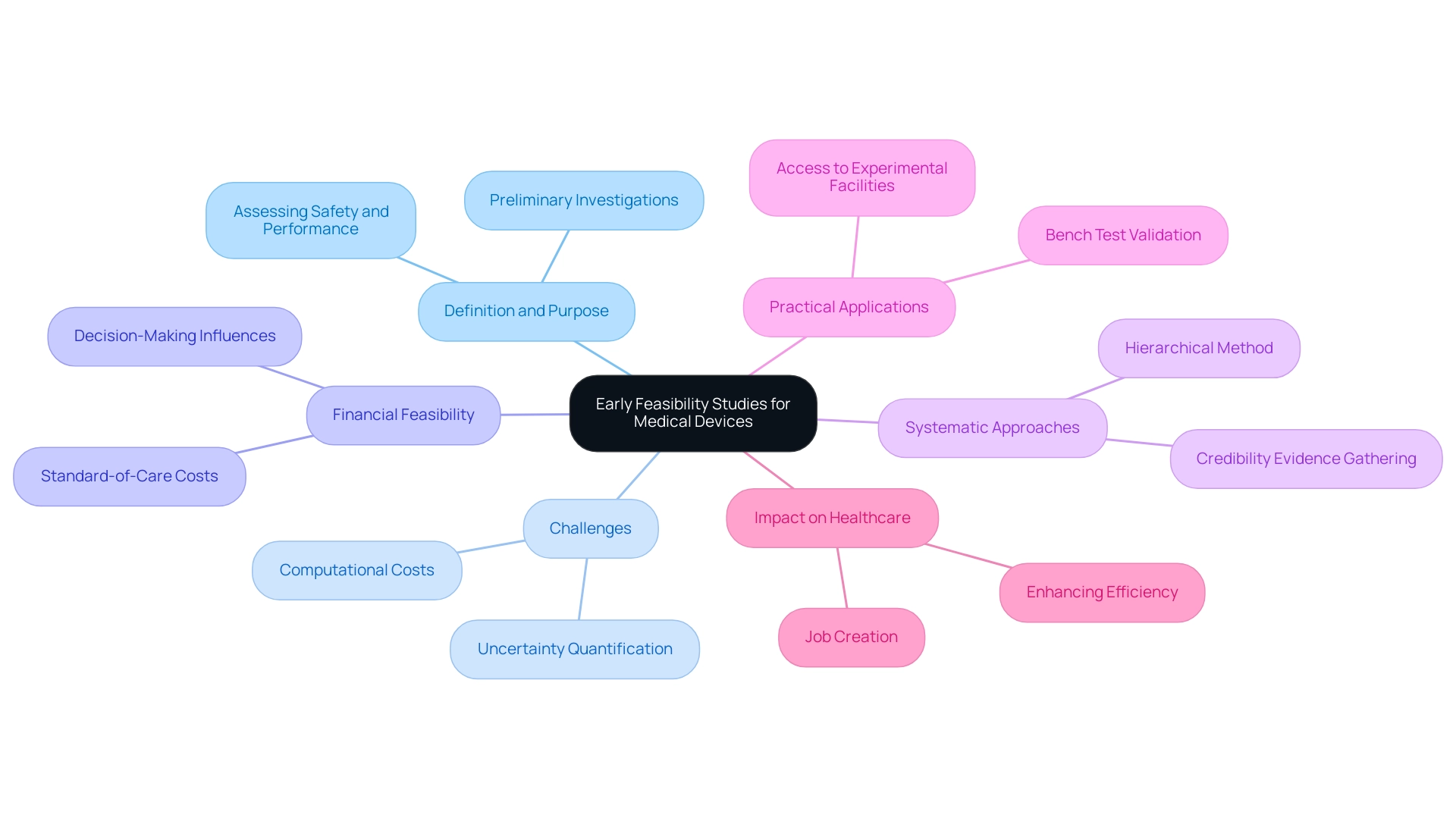
Understanding Early Feasibility Studies: Definition and Purpose
Early Feasibility Studies for Medical Devices in the form of initial feasibility assessments (EFS) function as crucial preliminary investigations intended to assess the practicality of innovative medical instruments before their advancement into full-scale clinical trials. A notable example of Early Feasibility Studies for Medical Devices in the field is ReGelTec’s recent study on HYDRAFIL™ for treating chronic low back pain, which successfully treated eleven patients in Barranquilla, Colombia, using remotely proctored procedures. This highlights how Early Feasibility Studies for Medical Devices in assessing the safety and performance of devices within a controlled patient population can ultimately inform design modifications and regulatory strategies.
Furthermore, these analyses significantly reduce the risks associated with later-stage trials. However, challenges such as high computational costs for calculation verification and uncertainty quantification (UQ) in an Integrated Simulation and Computational Testing (ISCT) framework can impact the feasibility and planning of EFS. Additionally, financial feasibility varies widely, influencing the decision-making process for clinical research directors.
Kenneth I. Aycock emphasizes the significance of a systematic approach to establishing the credibility of these analyses, proposing a hierarchical method for assessing ISCT credibility, which is critical for identifying potential issues early in the process. Practical applications, such as the case analysis on bench test validation outcomes, further demonstrate the necessity of access to physical equipment and experimental facilities, which can only occur after prototype units are produced. Ultimately, Early Feasibility Studies for Medical Devices in play a pivotal role in enhancing the overall efficiency of medical equipment development, ensuring patient safety, and improving the likelihood of success in subsequent clinical trials.
Furthermore, organizations such as bioaccess® offer vital knowledge in overseeing these projects, aiding local economies through job creation and enhancements in healthcare.
Key Components of Early Feasibility Studies: Risk Assessment and Technology Validation
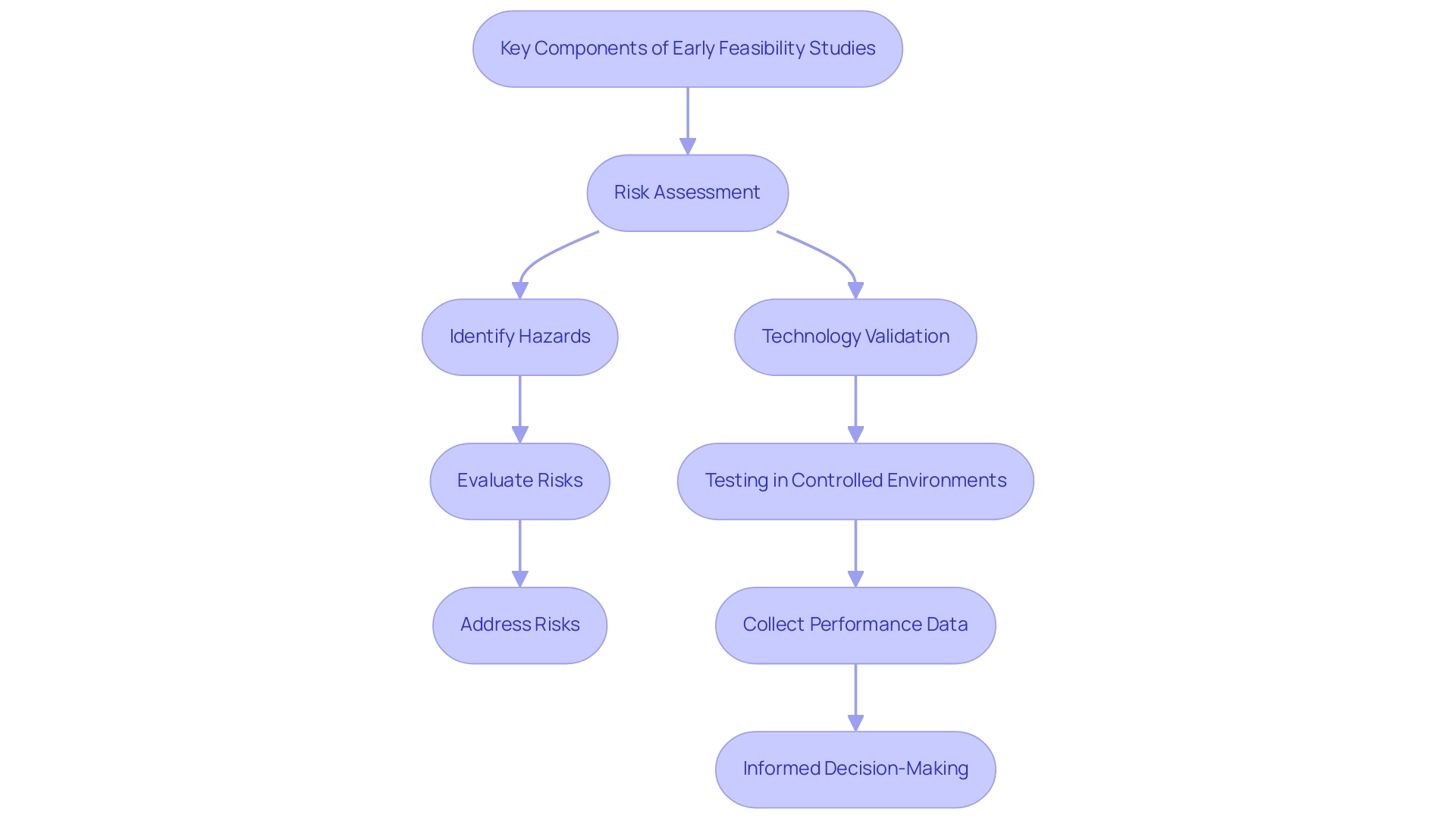
Early feasibility studies for medical devices in the development of medical equipment are essential, focusing primarily on risk assessment and technology validation. Risk assessment involves a systematic process of identifying potential hazards associated with the medical equipment and evaluating the likelihood and impact of these risks on patient safety. This thorough evaluation is essential for addressing significant risks prior to advancing to larger clinical trials, thereby safeguarding patient well-being and ensuring compliance with regulatory standards.
In 2024, navigating complex data protection laws remains pivotal for healthcare organizations, emphasizing the importance of compliance amid evolving identity protection expectations. As one expert noted, 'Ensuring privacy and compliance with growing identity protection expectations continues to be a concern for healthcare organizations.' Furthermore, the RN Recruitment Difficulty Index has risen to an average of 95 days, highlighting challenges in recruiting seasoned staff, which can negatively affect feasibility assessments and their implications on patient safety and equipment effectiveness.
Technology validation supports this process by verifying that the apparatus functions as intended and conforms to its design specifications. This validation is accomplished through rigorous testing in controlled environments, allowing researchers to collect preliminary performance data crucial for informed decision-making regarding its further development. Recent advancements in technology validation have shown promising success rates in Early Feasibility Studies for Medical Devices in reinforcing its significance in the medical equipment lifecycle.
By utilizing these methodologies, organizations can improve their research strategies. A relevant example is the CORE–MD project, started in April 2021, which seeks to enhance methods for the medical examination and assessment of high-risk medical instruments throughout Europe. This partnership among oversight agencies, public health institutes, and academic institutions highlights the increasing acknowledgment of the necessity for strong validation processes and effective risk mitigation strategies in the medical device sector.
At bioaccess®, we specialize in comprehensive trial management services, including Early Feasibility Studies for Medical Devices in addition to First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). With more than 20 years of experience in Medtech, our team provides a tailored approach to guide your company through the compliance environment for Early Feasibility Studies for Medical Devices in early-feasibility and first-in-human trials.
Navigating Regulatory Requirements for Early Feasibility Studies
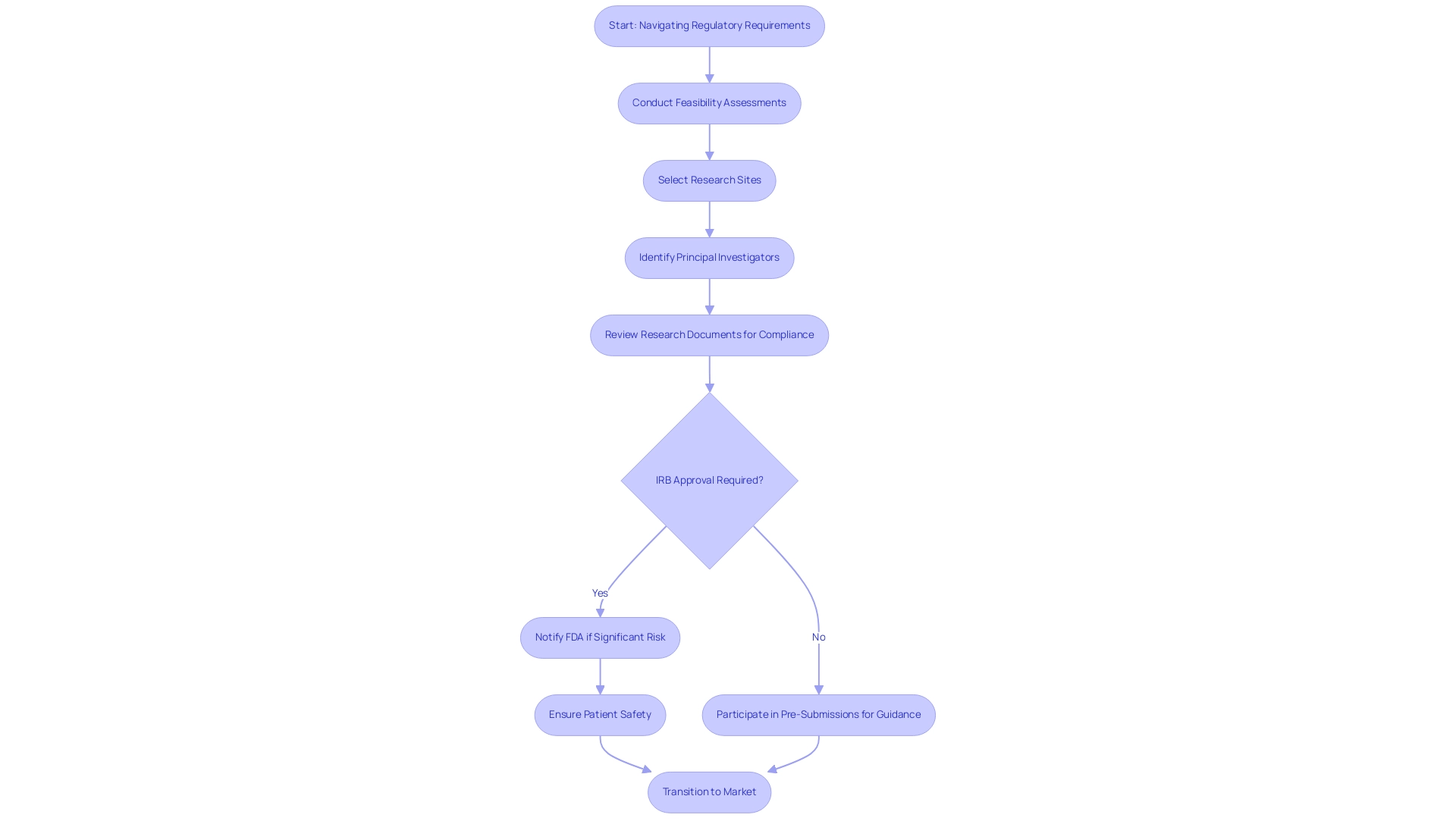
Navigating the regulatory landscape for Early Feasibility Studies for Medical Devices in Latin America is a crucial element of the clinical research process. Our comprehensive services facilitate this journey, encompassing:
- Feasibility assessments
- The selection of research sites
- The identification of principal investigators (PIs) to ensure effective project execution
We provide thorough review and feedback on research documents to comply with country requirements, ensuring that all necessary protocols are followed.
Regulatory compliance is paramount; for instance, the requirement for Institutional Review Board (IRB) approval plays a critical role in safeguarding participant rights and welfare. In cases where an IRB categorizes a research project as significant risk, prompt notification to the FDA is necessary before commencement, underscoring the importance of adhering to regulatory protocols to protect patient safety and enhance research credibility. In the U.S., the FDA supervises these investigations and offers guidelines that encompass design, data collection, and reporting practices.
Adhering to these regulations not only guarantees compliance but also aids in a smoother transition into later phases, ultimately accelerating the journey to market for innovative medical devices. Furthermore, the FDA encourages researchers to participate in Pre-Submissions, allowing them to submit critical information and receive guidance on non-clinical testing plans and draft clinical protocols. This proactive method encourages cooperation between researchers and oversight organizations, ensuring that investigations are structured to fulfill current compliance expectations.
Staying informed about the latest compliance updates, including those for 2024, is vital for successfully navigating the complexities associated with early feasibility studies for medical devices. With experts like Katherine Ruiz leading the way in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, navigating these challenges becomes a streamlined process. For further assistance, we invite you to schedule a meeting to discuss how we can support your trial needs.
Challenges in Conducting Early Feasibility Studies: Overcoming Obstacles
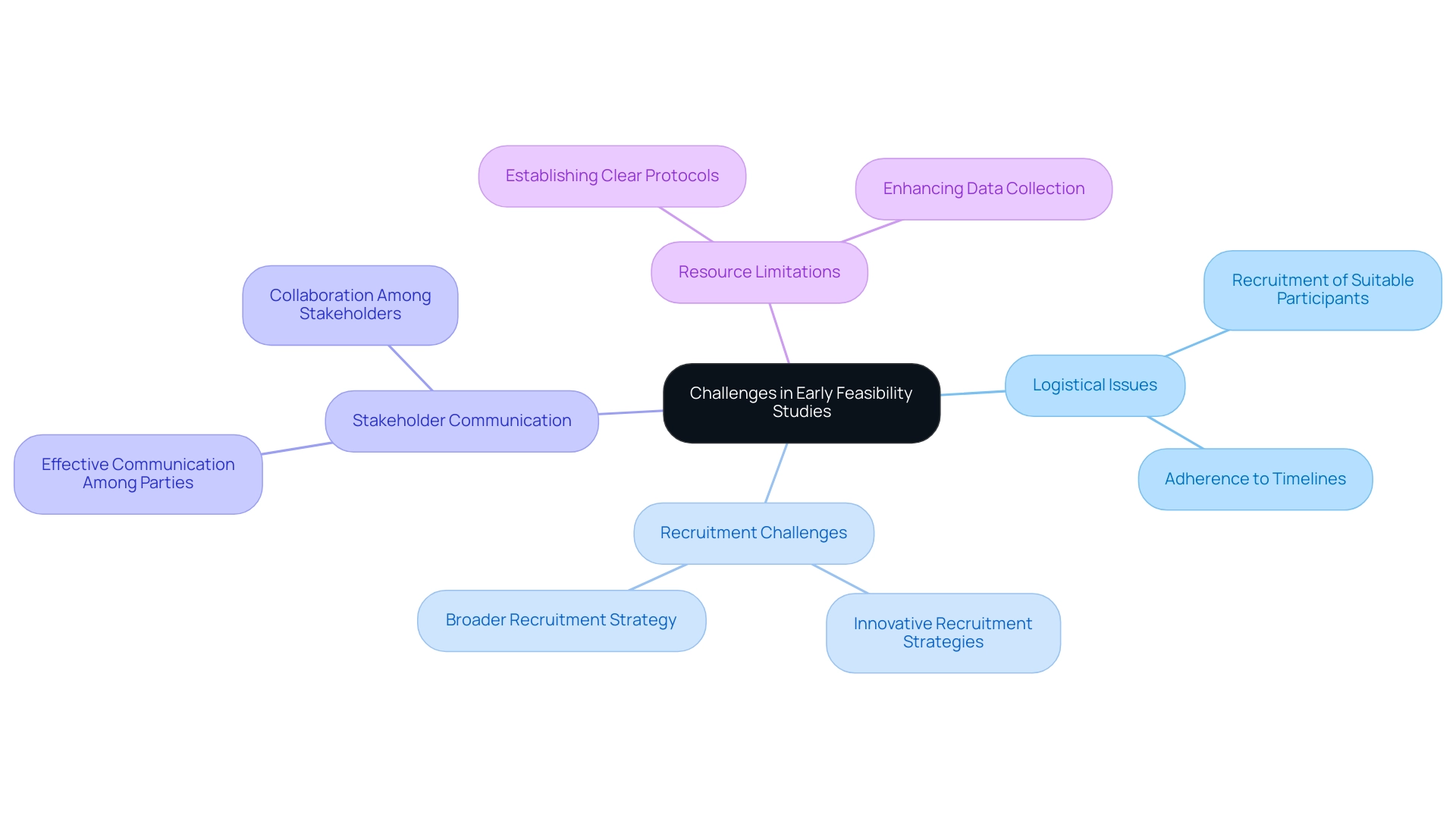
Early feasibility studies for medical devices in the Latin American environment introduce a variety of substantial challenges that can affect the overall success of research. Logistical issues, such as the recruitment of suitable participants and adherence to timelines, often emerge as primary obstacles. Recent statistics from 2024 indicate that only 30% of medical trials meet their recruitment goals, underscoring participant recruitment as a critical hurdle.
Reports highlight the necessity for innovative strategies to improve recruitment efforts in research trials. Involving stakeholders—including sponsors, regulatory bodies, and research teams—is essential, yet it can often be filled with challenges. Dr. Valentin Fuster, Editor-in-Chief, states, "Effective communication among all parties involved is essential to streamline processes and ensure success in clinical research."
Researchers must also navigate the delicate balance between the necessity for comprehensive data collection and the limitations posed by available resources. To address these challenges, establishing clear protocols and timelines is essential. Such measures not only enhance the efficiency of the research but also facilitate collaboration among team members and stakeholders, paving the way for successful outcomes in Early Feasibility Studies for Medical Devices in assessments.
Furthermore, bioaccess® brings over 20 years of expertise in managing various types of research, including:
- Early-Feasibility Research (EFS)
- First-In-Human Research (FIH)
- Pilot Research
- Pivotal Research
- Post-Market Clinical Follow-Up Research (PMCF)
This expertise is essential for navigating complex compliance environments. Bioaccess® utilizes a tailored approach that adjusts to the distinct requirements of each project, ensuring flexibility in addressing challenges that arise.
Case analyses illustrate that enhancing patient care through innovative technologies, highlighted by the Early Feasibility Studies for Medical Devices in the FDA's Early Feasibility Initiative, emphasizes the societal necessity of improving oversight processes through stakeholder collaboration. This program illustrates how effective collaboration can lead to better patient outcomes and more efficient regulatory pathways.
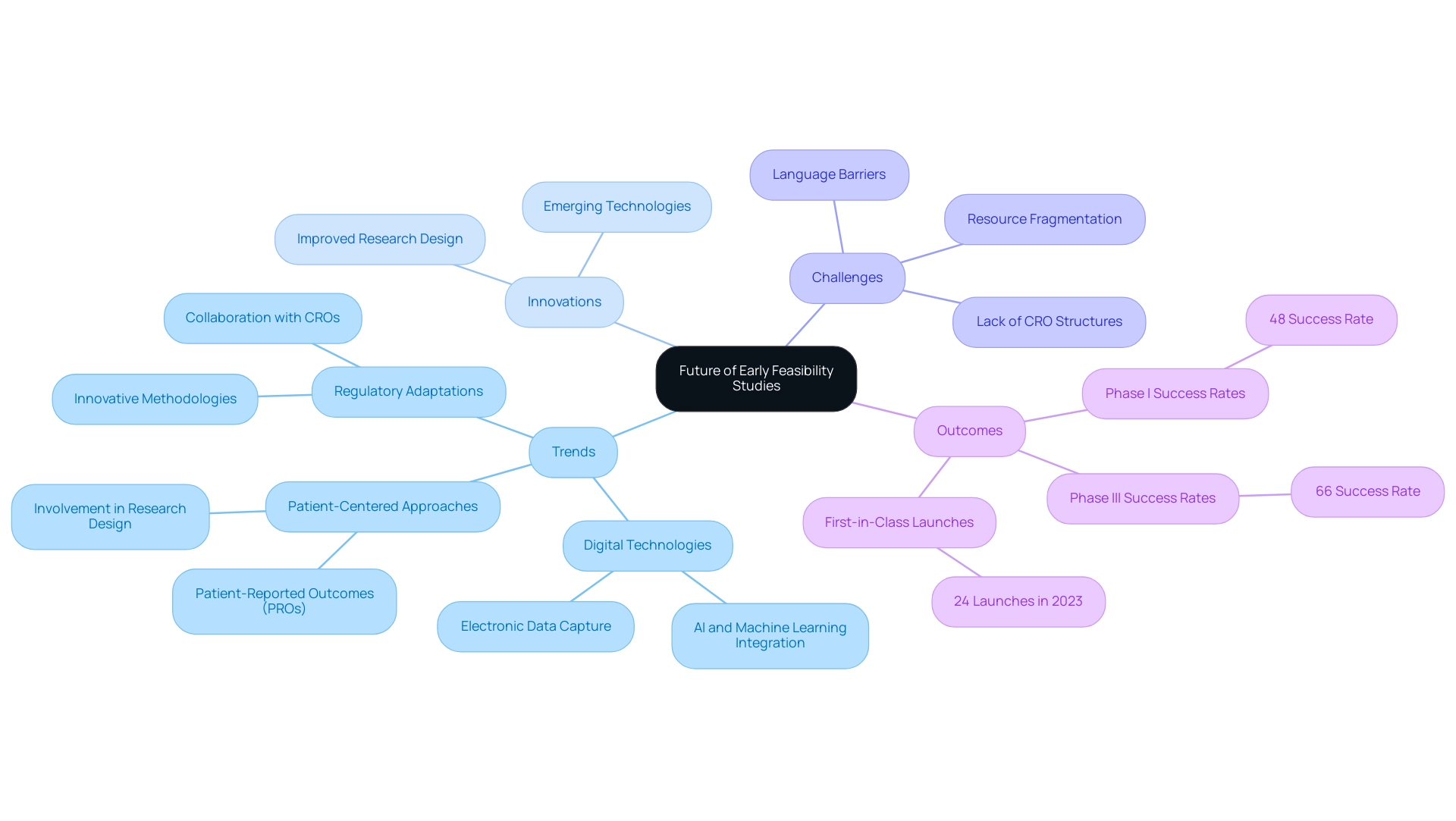
The Future of Early Feasibility Studies: Trends and Innovations
Early feasibility studies for medical devices in Latin America are rapidly evolving, driven by emerging trends and innovations aimed at improving the efficiency and effectiveness of medical device development. A significant trend is the increasing integration of digital technologies, particularly artificial intelligence (AI) and machine learning, which are revolutionizing data analysis and improving research design. These advancements enable researchers to process large datasets with unprecedented speed and accuracy, facilitating more informed decision-making.
Furthermore, a notable shift towards patient-centered approaches in medical research is emerging. Involving patients in the design and execution of research ensures that medical devices align with their needs and preferences, ultimately enhancing the relevance and utility of research outcomes. This is demonstrated by the case analysis on patient-reported outcomes (Pros), where electronic capture methods (ePROs) offer stronger and more precise data, enhancing the reliability of results in research.
However, Medtech companies in Latin America continue to face challenges such as:
- Language barriers
- Fragmentation of resources
- A lack of established CRO structures, which complicate the execution of trials.
Regulatory bodies in the region are adapting to these advancements, paving the way for the incorporation of innovative methodologies and technologies in early assessments. As highlighted by Dr. Sergio Alvarado, a Clinical Trial Manager focused on innovative medical research and artificial intelligence in the region, this evolution underscores the collaboration between Greenlight Guru and bioaccess® to accelerate Medtech innovations and clinical trials.
In 2023, the industry saw 24 first-in-class launches in the U.S., a testament to the growing impact of these trends. Additionally, Phase I success rates rose to 48%, while Phase III rates increased to 66%, exceeding the 10-year pre-pandemic average, further illustrating enhancements in results. As we look towards 2024, the emphasis on digital technologies and patient engagement is expected to continue shaping the future of early feasibility studies, providing opportunities for innovation and improved patient outcomes, while addressing the urgent need for a solution-driven approach to bridge existing gaps.
Conclusion
The exploration of early feasibility studies (EFS) underscores their vital role in the development of innovative medical devices, particularly in the context of Latin America. By assessing the viability of new technologies, these studies not only enhance patient safety but also ensure compliance with regulatory requirements, ultimately paving the way for successful clinical trials. The key components of EFS, including rigorous risk assessment and comprehensive technology validation, are essential for identifying potential hazards and confirming device performance, thereby reinforcing the integrity of the medical device lifecycle.
Despite the numerous advantages that EFS provide, challenges such as participant recruitment and logistical hurdles remain prevalent. The statistics indicating that only 30% of clinical trials meet their recruitment goals highlight the necessity for innovative strategies to engage stakeholders effectively. Moreover, the evolving regulatory landscape requires continuous adaptation and collaboration between researchers and regulatory bodies to navigate complexities and ensure adherence to protocols.
Looking ahead, the future of early feasibility studies is poised for transformation through the integration of digital technologies and a patient-centered approach. Innovations such as artificial intelligence and electronic capture methods are revolutionizing data analysis and enhancing study design, leading to improved outcomes. As the industry continues to evolve, the emphasis on collaboration and innovative methodologies will be crucial in addressing existing challenges and driving advancements in medical device research.
In conclusion, understanding the intricacies of early feasibility studies is essential for stakeholders aiming to enhance clinical investigation strategies. By prioritizing these studies, organizations can significantly improve patient safety, optimize regulatory compliance, and ultimately contribute to the successful development of medical innovations that meet the needs of the healthcare landscape.
Frequently Asked Questions
What are Early Feasibility Studies (EFS) for medical devices?
Early Feasibility Studies (EFS) are preliminary investigations designed to assess the practicality of innovative medical devices before they progress to full-scale clinical trials. They evaluate the safety and performance of devices within a controlled patient population.
Can you provide an example of an Early Feasibility Study?
A notable example is ReGelTec’s study on HYDRAFIL™ for treating chronic low back pain, which successfully treated eleven patients in Barranquilla, Colombia, using remotely proctored procedures.
What are the benefits of conducting Early Feasibility Studies?
EFS significantly reduce risks associated with later-stage trials, inform design modifications, and help develop regulatory strategies, ultimately enhancing patient safety and increasing the likelihood of success in subsequent clinical trials.
What challenges do Early Feasibility Studies face?
Challenges include high computational costs for calculation verification, uncertainty quantification in Integrated Simulation and Computational Testing (ISCT), and varying financial feasibility, which can influence clinical research decisions.
How does risk assessment factor into Early Feasibility Studies?
Risk assessment involves identifying potential hazards associated with medical equipment and evaluating their likelihood and impact on patient safety. This thorough evaluation is critical for addressing significant risks before advancing to larger clinical trials.
What role does technology validation play in Early Feasibility Studies?
Technology validation verifies that the medical device functions as intended and meets design specifications through rigorous testing in controlled environments, providing preliminary performance data essential for informed decision-making.
What is the CORE–MD project?
The CORE–MD project, initiated in April 2021, aims to enhance methods for the examination and assessment of high-risk medical instruments across Europe, demonstrating the importance of strong validation processes and effective risk mitigation strategies.
What services does bioaccess® offer in relation to Early Feasibility Studies?
Bioaccess® provides comprehensive trial management services, including Early Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies, drawing on over 20 years of experience in the Medtech field.




