Introduction
In the realm of clinical research, the transition from traditional data collection methods to Electronic Data Capture (EDC) systems marks a pivotal advancement that enhances the integrity and efficiency of clinical trials. These sophisticated digital platforms not only streamline data management but also integrate seamlessly with various clinical trial management services, ensuring compliance with rigorous regulatory standards.
As the industry witnesses a growing trend towards mobile EDC solutions and virtual data capture, it becomes increasingly vital for stakeholders to understand the myriad benefits, challenges, and innovations associated with these systems.
This article delves into the essential features of EDC systems, the significant advantages they offer, and the future trends shaping their evolution, providing a comprehensive overview for organizations navigating this transformative landscape.
Understanding Electronic Data Capture (EDC) Systems in Clinical Trials
EDC systems clinical trials signify a notable advancement in the collection, management, and storage of clinical study data, seamlessly integrating with comprehensive clinical study management services. Our capabilities include:
- Conducting feasibility studies
- Selecting appropriate research sites
- Ensuring compliance reviews
These are all essential for effective setup and operation. This includes obtaining ethics committee approval and import permits, which are crucial for the initiation of clinical trials.
These advanced digital platforms streamline the information collection process, offering functionalities such as:
- Cart additions
- Loading indicators
These features enhance user experience and efficiency. By facilitating real-time access and oversight, EDC systems clinical trials enable researchers to collect information from various locations precisely and reliably, which is essential considering the anticipated 16% yearly growth rate for mobile EDC solutions. A major trend in the electronic data collection market is the emergence of virtual data capture in clinical trials, highlighting the industry's shift towards more innovative EDC systems clinical trials.
Furthermore, the compliance with regulatory standards in the tightly regulated landscape of clinical research, overseen by entities like INVIMA, is crucial and is significantly supported by EDC systems clinical trials, which also handle medical device classification and regulatory functions in Colombia. Key players in the EDC solutions market, including IBM, Oracle, IQVIA, Parexel, and Veeva Systems, are instrumental in shaping the market landscape and driving innovation in EDC solutions. The shift from conventional paper-based techniques to EDC systems clinical trials not only streamlines information handling but also enhances the overall integrity and dependability of clinical study results.
Benefits of Implementing EDC Systems in Clinical Research
The execution of EDC systems clinical trials in clinical research offers substantial benefits, including improved information accuracy, heightened operational efficiency, and better regulatory compliance. Our comprehensive clinical trial management services cover all critical areas, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting on study status, including serious and non-serious adverse events
By greatly minimizing the possibility of human mistakes associated with manual information input, EDC systems for clinical trials guarantee that the integrity of the information is preserved.
A recent meta-analysis utilizing the Freeman-Tukey transformation method and generalized linear mixed models indicates that these frameworks contribute to lower error rates in information processing compared to traditional methods, further emphasizing their effectiveness in enhancing accuracy. Additionally, the use of EDC systems in clinical trials results in significant cost reductions throughout a clinical study by reducing manual information entry and document handling. The ability to access and analyze information in real-time empowers researchers to make prompt, informed decisions, thereby streamlining the trial process.
Additionally, EDC systems clinical trials are equipped with built-in compliance checks, supporting adherence to stringent regulatory standards such as FDA 21 CFR Part 11, which helps mitigate the risk of costly delays and penalties. The importance of audit trails in EDC systems clinical trials cannot be overlooked; they meticulously document every data change, including the identity of the individual who made the change and the timestamp, which is critical for upholding data integrity and ensuring compliance with regulatory requirements. Katherine Ruiz, our expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, plays a crucial role in ensuring that our processes align with these standards.
By ensuring robust management of clinical trials, we contribute not only to successful study outcomes but also to job creation, economic growth, and improved healthcare in local communities.
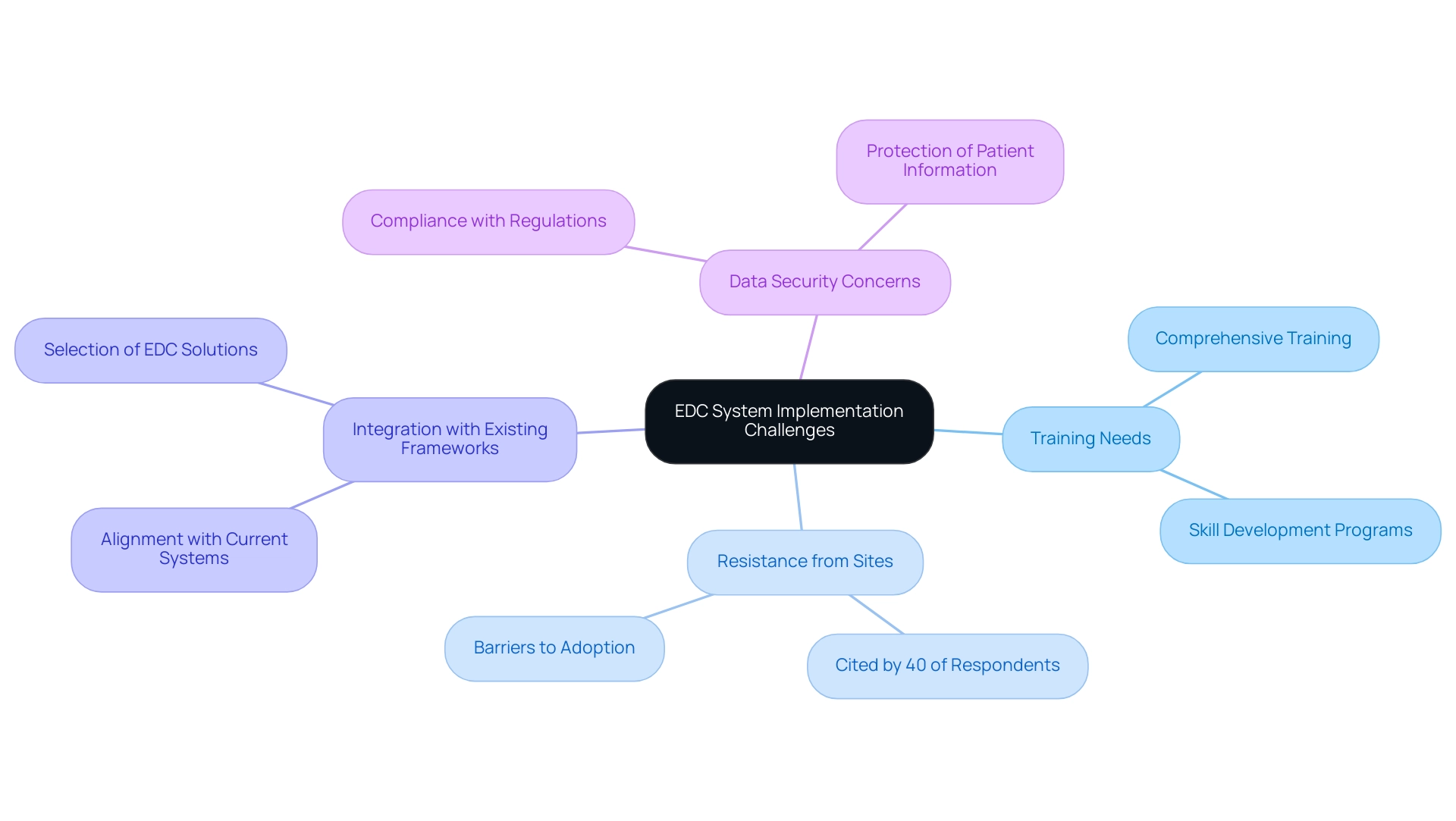
Challenges and Considerations in EDC System Implementation
The implementation of Electronic Data Capture (EDC) tools, while beneficial, is often faced with substantial challenges. A notable concern is the necessity for comprehensive training for site staff, which is critical for the effective utilization of these tools. Resistance from investigative sites has been identified as a major barrier to EDC adoption, with 40% of respondents citing this issue as a key factor in delays.
Organizations must therefore allocate adequate time and resources to equip personnel with the necessary skills. A relevant case study titled 'Implementing Electronic Data Capture Training for Site Staff' highlights the importance of EDC training programs designed to enhance user proficiency. This initiative aimed to enhance data precision and productivity in clinical studies, demonstrating the direct correlation between well-trained personnel and the successful execution of EDC frameworks.
As Henry J. noted, "Adoption of Electronic Health Record Systems Among U.S. Non-Federal Acute Care Hospitals: 2008–2015," emphasizes the critical nature of training in technology adoption.
Additionally, integration with existing frameworks presents another challenge, requiring a careful selection of EDC solutions that align with current organizational infrastructures. Data security remains an overarching concern, particularly given the sensitive nature of patient information involved in clinical trials. It is essential that organizations select EDC solutions that comply with strict protection regulations and include strong security measures to avert potential breaches.
While the challenges are significant, it is essential to recognize that the benefits of EDC outweigh these challenges. As the landscape of clinical research evolves, continual reassessment and re-evaluation of processes related to EDC platforms will be essential to maximize their benefits and mitigate associated challenges.
Future Trends and Innovations in EDC Systems
The evolution of EDC systems clinical trials is set to undergo significant changes driven by the integration of advanced technologies such as artificial intelligence (AI) and blockchain. AI is transforming information analysis capabilities, enabling predictive analytics that can improve study design and simplify patient recruitment strategies, essential for effective feasibility assessments and site selection. This advancement optimizes the clinical study process and empowers researchers to make data-driven decisions with greater accuracy.
Significantly, North America was the largest area in the electronic information capture systems market in 2023, emphasizing the region's crucial role in this changing environment. Simultaneously, blockchain technology is rising as a potent instrument for enhancing information integrity and security, ensuring that details gathered during clinical assessments are trustworthy and transparent—crucial for compliance evaluations. As Steve Bennett, a Business Formation Specialist, mentions, 'This article intends to highlight the key trends and statistics of EDC software in 2024, offering stakeholders the insights required to navigate and thrive in the constantly changing realm of information capture.'
Moreover, the thorough administration of clinical studies, including study setup, import permits, and project management, is increasingly enhanced by the rise of virtual information collection. This reflects the industry's shift towards more flexible and efficient data collection methods, ultimately contributing to job creation, economic growth, and healthcare improvement in local economies. The review and feedback on study documents to comply with country requirements, along with the import permit and nationalization of investigational devices, are critical components of this process.
As these technologies continue to develop, they are expected to further transform the clinical trial landscape, particularly with the use of edc systems clinical trials, fostering a new era of innovation in the field.
Key Features to Consider When Choosing an EDC System
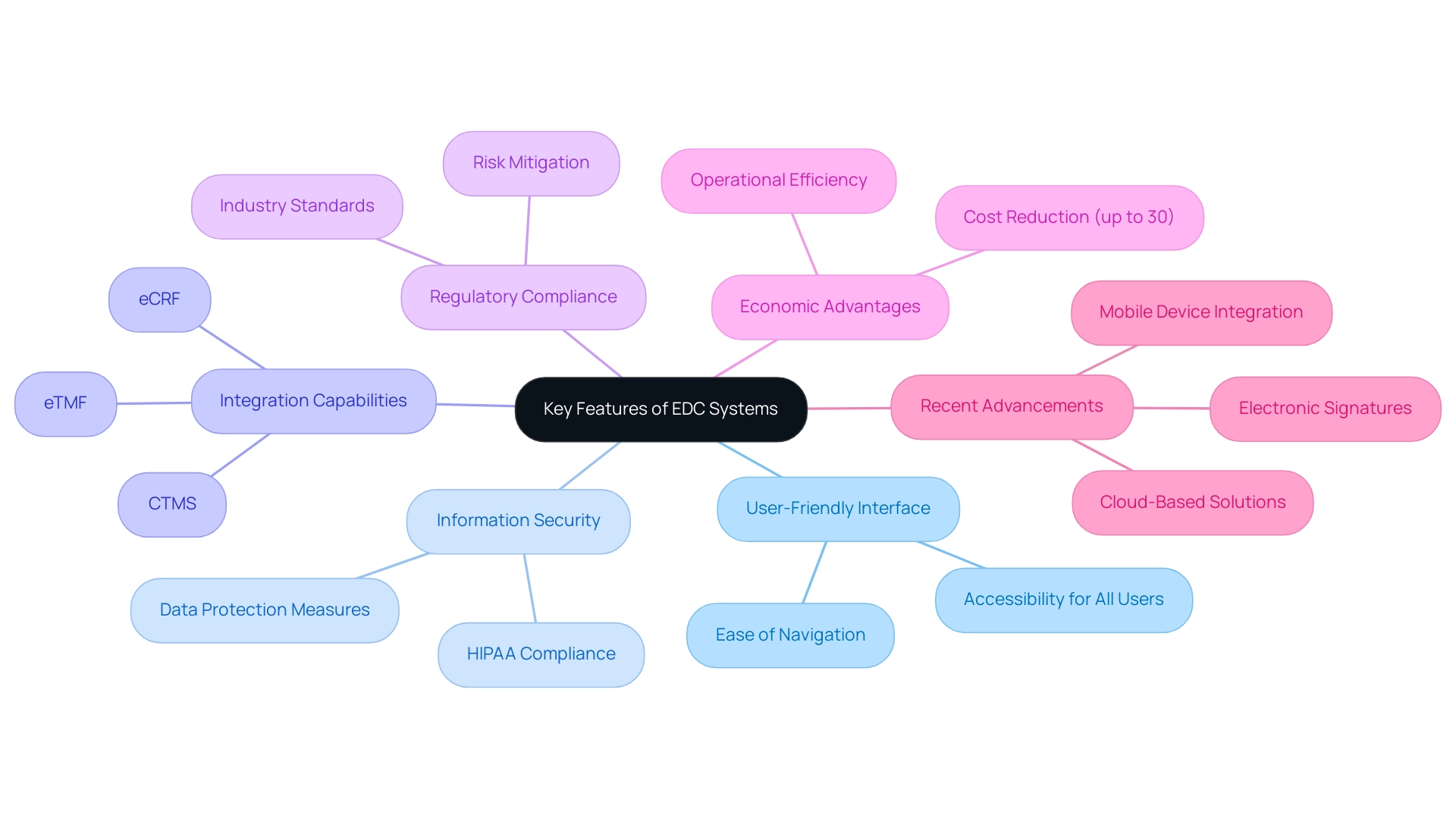
When choosing an Electronic Data Capture (EDC) solution, organizations must carefully assess several essential features. A user-friendly interface is paramount, as it ensures that all team members, regardless of their technical expertise, can navigate the system efficiently. This accessibility can significantly enhance the overall productivity of EDC systems in clinical trials.
Furthermore, robust information security features are critical to protect sensitive details, particularly in compliance with HIPAA regulations, thereby safeguarding patient confidentiality. Integration capabilities are also vital; the EDC platform should seamlessly connect with existing software, including:
- electronic Case Report Forms (eCRF)
- Clinical Trial Management Systems (CTMS)
- electronic Trial Master Files (Etmf)
to maintain workflow continuity and enhance interoperability. Lastly, regulatory compliance cannot be overlooked; an EDC solution must meet industry standards to mitigate risks associated with data management, ultimately ensuring the integrity and reliability of research data.
Significantly, EDC systems in clinical trials can lower operational expenses by as much as 30%, emphasizing the economic advantages of embracing such platforms. As Cynthia Haydon from the University of Missouri School of Medicine emphasizes, these features collectively contribute to the operational efficiency and success of EDC systems in clinical trials. Recent advancements in EDC systems, as discussed in the case study 'Modern Developments in Electronic Data Capture,' illustrate how integrating mobile devices, electronic signatures, and cloud-based solutions enhances data collection and improves patient engagement.
Conclusion
The transition to Electronic Data Capture (EDC) systems represents a transformative shift in clinical research, enhancing data management, accuracy, and regulatory compliance. By replacing traditional paper-based methods with sophisticated digital platforms, organizations can significantly reduce human error, streamline operations, and improve the integrity of clinical trial outcomes. The integration of advanced technologies, such as artificial intelligence and blockchain, further positions EDC systems at the forefront of innovation, promising enhanced data analysis and security.
Despite the numerous advantages, the implementation of EDC systems is not without its challenges. Training for site staff and integration with existing infrastructures are critical considerations that organizations must address to maximize the potential of these systems. Acknowledging and overcoming these obstacles is essential to fully leverage the benefits that EDC systems offer.
As the clinical research landscape continues to evolve, staying informed about future trends and innovations within EDC systems is paramount. Organizations must prioritize the selection of user-friendly, secure, and compliant EDC solutions that align with their operational needs. By doing so, they can not only improve the efficiency of clinical trials but also contribute to the overall growth and advancement of healthcare outcomes. Embracing this digital transformation will ultimately pave the way for more effective and reliable clinical research, benefitting stakeholders and patients alike.
Frequently Asked Questions
What are EDC systems in clinical trials?
EDC (Electronic Data Capture) systems in clinical trials are advanced digital platforms designed to streamline the collection, management, and storage of clinical study data, integrating with comprehensive clinical study management services.
What capabilities do EDC systems provide for clinical trials?
EDC systems offer capabilities such as conducting feasibility studies, selecting appropriate research sites, ensuring compliance reviews, trial setup, obtaining import permits, project management, and reporting on study status, including adverse events.
How do EDC systems improve the clinical trial process?
EDC systems enhance the clinical trial process by improving information accuracy, increasing operational efficiency, reducing manual data entry errors, and enabling real-time access to data, which facilitates informed decision-making.
What are some key features of EDC systems?
Key features of EDC systems include cart additions, loading indicators, built-in compliance checks, and audit trails that document every data change, ensuring data integrity and regulatory compliance.
Why is compliance important in clinical trials?
Compliance is crucial in clinical trials to adhere to stringent regulatory standards, such as FDA 21 CFR Part 11, which helps mitigate risks of costly delays and penalties, ensuring the integrity of the data collected.
What trends are emerging in the EDC systems market?
A major trend in the EDC systems market is the shift towards virtual data capture in clinical trials, reflecting the industry's move towards more innovative and efficient electronic data collection methods.
Who are the key players in the EDC solutions market?
Key players in the EDC solutions market include IBM, Oracle, IQVIA, Parexel, and Veeva Systems, which are instrumental in driving innovation and shaping the market landscape.
How do EDC systems contribute to cost reductions in clinical trials?
EDC systems contribute to cost reductions by minimizing manual information entry and document handling, which lowers error rates and streamlines the overall trial process, leading to significant savings throughout a clinical study.
What role does Katherine Ruiz play in the context of EDC systems?
Katherine Ruiz is an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, ensuring that the processes related to EDC systems align with regulatory standards.




