Introduction
The integration of human factors in medical device design is a pivotal area of focus that directly influences patient safety and device efficacy. As the healthcare landscape evolves, understanding how users interact with medical technologies becomes increasingly essential. This article delves into the multifaceted aspects of human factors, exploring their impact on usability, regulatory compliance, and design innovation.
It highlights the importance of user-centered design principles and the need for comprehensive usability testing to mitigate potential errors. Through case studies and expert insights, the discussion underscores the significance of aligning medical device development with user needs, ultimately aiming for improved outcomes in clinical settings.
As challenges persist in this dynamic field, the future of human factors engineering promises to harness technological advancements for enhanced user experiences and device performance.
Understanding Human Factors in Medical Device Design
The design of medical equipment incorporates human factors in medical device design, focusing on the thorough examination of interactions between individuals and systems, including aspects such as ergonomics, cognitive psychology, and user-focused design principles. The significance of human factors in medical device design is highlighted by their direct influence on the safety, effectiveness, and overall experience of medical equipment. A comprehensive grasp of human factors in medical device design, including how individuals perceive, interact with, and utilize these tools, enables designers to develop products that significantly lower the probability of mistakes, thereby enhancing usability and improving patient outcomes.
This interdisciplinary approach not only ensures that medical instruments are technically robust but also that they are intuitively designed and user-friendly. By prioritizing the needs and preferences of healthcare professionals and patients, the development process can yield devices that facilitate not just functionality but also optimal engagement of individuals. Recent studies, including those conducted within high-risk industries, highlight the relevance of alarm formulation and usage guidelines, emphasizing the necessity for clarity and simplicity in alerts to foster effective responses from users.
Significantly, 110 of 110 nuclear industry guidelines are pertinent, reinforcing the significance of alarm principles. For example, the automotive and nuclear sectors suggest creating alarms to be simple and comprehensible, with redundancy in bimodal alarms, as outlined in case studies on alarm construction characteristics. As expressed by Robert Rauschenberger, "The insights from clients regarding product performance in the field can be invaluable for innovation in creation and quality enhancement," reinforcing the essential feedback loop between client experience and creation improvement.
Applying Human Factors Engineering for Enhanced Usability
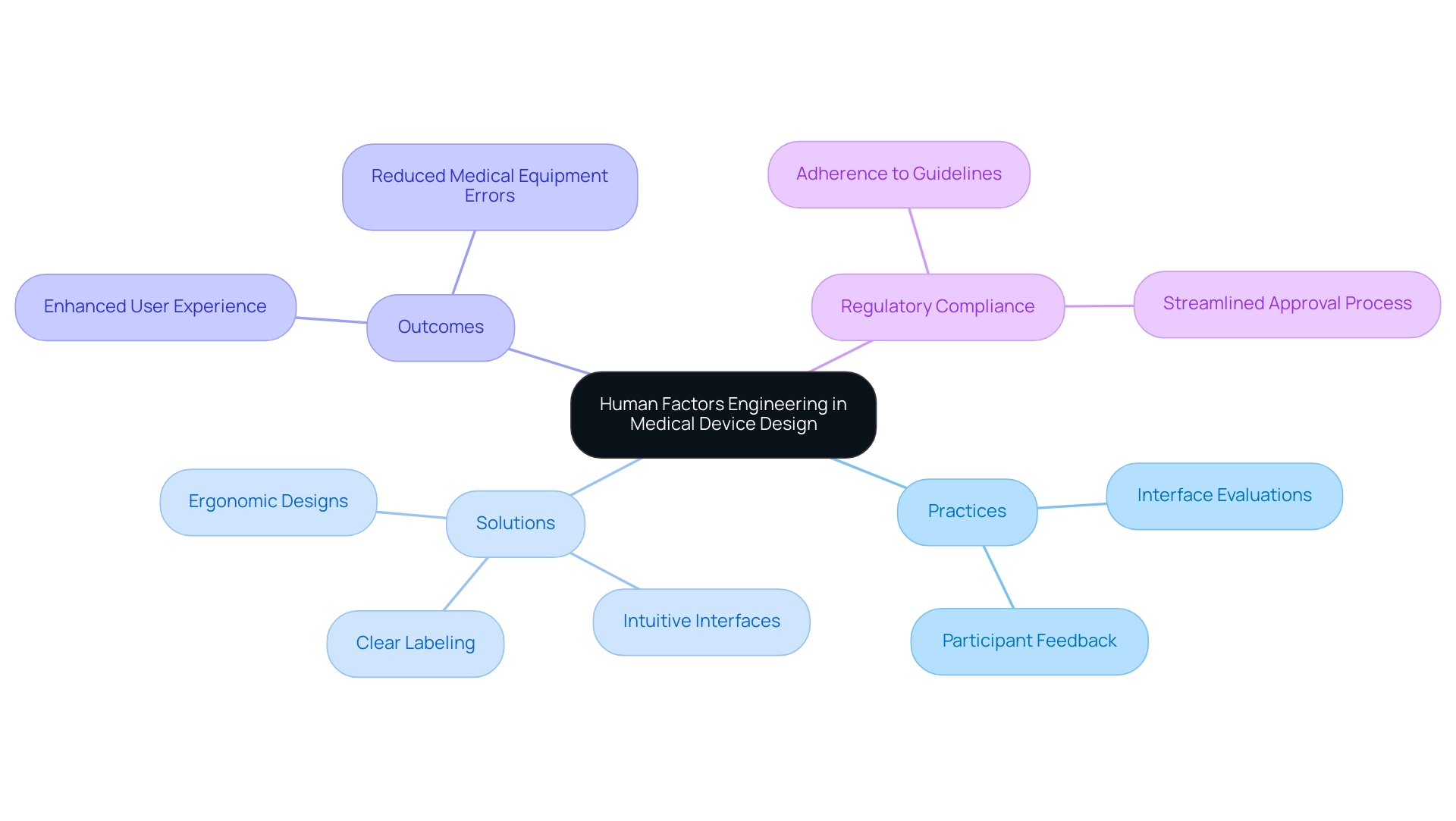
The creation and advancement of medical devices rely heavily on human factors in medical device design, as it directly addresses interaction elements that affect the individual. Performing comprehensive interface evaluations and collecting ongoing participant feedback are crucial practices that guide iterative enhancements based on real-world usage situations. The recent INFPUMP initiative revealed 107 functionality problems among 34 participants, highlighting the importance of recognizing challenges early in the development process.
By integrating human factors in medical device design, manufacturers can implement effective solutions such as:
- Clear labeling
- Intuitive interfaces
- Ergonomic designs
These solutions significantly enhance user experiences for both healthcare providers and patients. Additionally, the increase of telemedicine has made remote testing a viable option, allowing for broader participant recruitment in research studies. This trend not only expands the diversity of feedback but also enriches the development process.
Additionally, comparing the performance of matrix-based methods with other usability testing methodologies can provide insights into the most effective approaches for identifying usability issues. Adhering to regulatory guidelines that emphasize human factors in medical device design can streamline compliance during the approval process, ultimately facilitating faster market entry for innovative medical solutions. HFE principles are essential in decreasing medical equipment errors by incorporating human factors in medical device design, ensuring that products are designed with the end-consumer in mind and minimizing potential mistakes by individuals.
As Wade Schroeder, a Medical Device Guru at Greenlight Guru, aptly states,
Get feedback early and often.
This method is crucial for reducing medical equipment mistakes and ensuring that products satisfy the real requirements of individuals. Additionally, post-market monitoring and assessment must encompass the gathering and analysis of functionality data to consistently enhance performance and user satisfaction.
The Impact of Regulatory Standards on Human Factors in Design
Regulatory standards, particularly those outlined by the FDA and ISO, are crucial in influencing the incorporation of human factors in medical device design. These frameworks mandate that manufacturers perform comprehensive risk assessments and usability testing to pinpoint potential user-related errors. By strictly adhering to these regulations, companies not only maintain compliance but also enhance the safety and effectiveness of their products.
The FDA's guidance on the application of human factors engineering underscores the necessity of a user-centered design approach, enabling manufacturers to develop products that meet regulatory requirements while being tailored to user needs. This alignment with regulatory expectations not only instills confidence among stakeholders but also contributes significantly to improved patient outcomes.
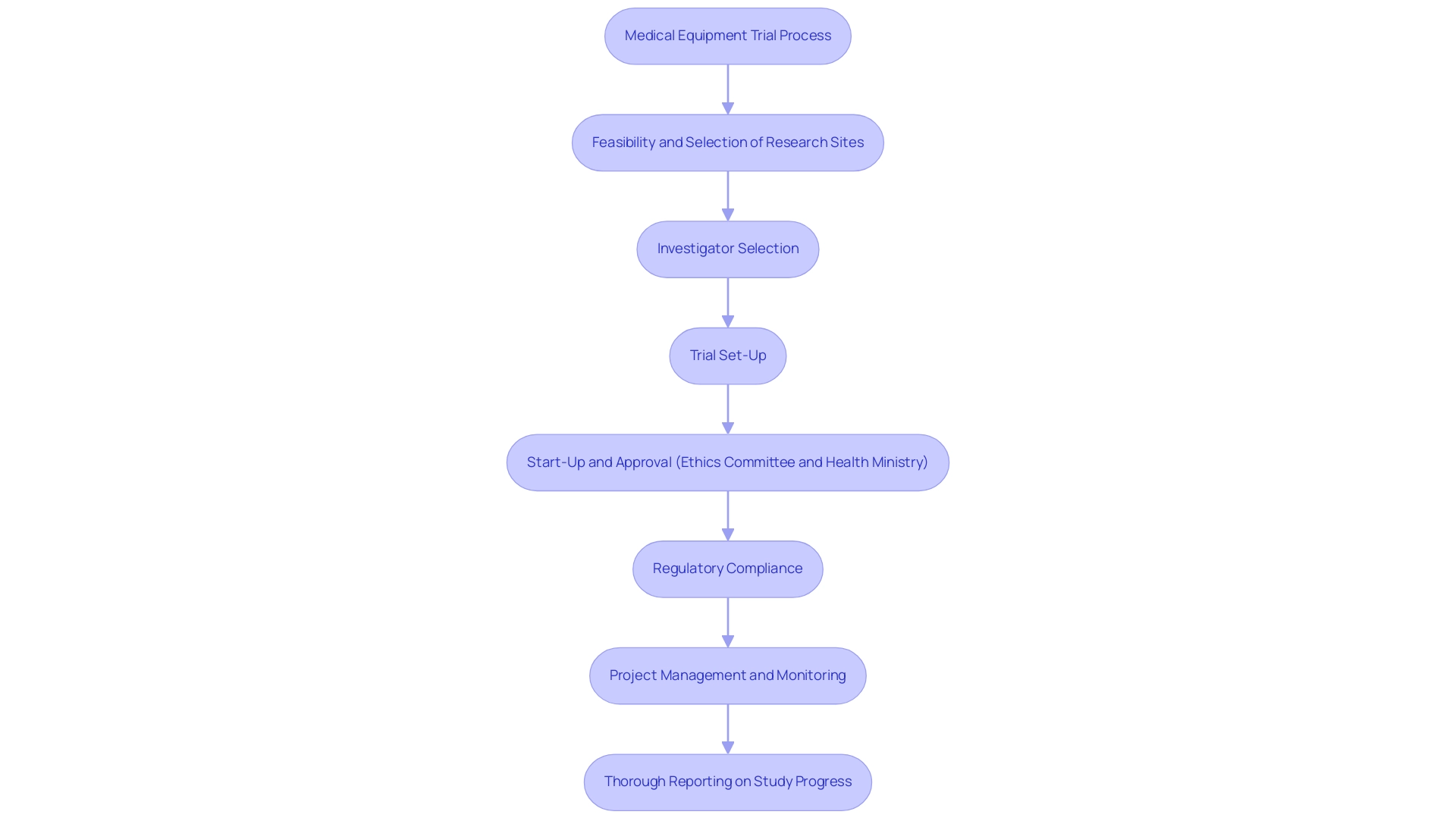
In the context of medical equipment trials, the services provided encompass:
- Feasibility and selection of research sites
- Investigator selection
- Trial set-up
- Start-up and approval (ethics committee and health ministry)
- Regulatory compliance
- Project management and monitoring
- Thorough reporting on study progress, including study status, inventory, and serious and non-serious adverse events
These components are critical for overcoming challenges faced by medical equipment startups, including regulatory hurdles and recruitment issues. Furthermore, the FDA emphasizes that any data from clinical investigations must be approved and reviewed by an Institutional Review Board (IRB), ensuring rigorous oversight in the development process.
As stated by the FDA, 'Except as provided in §§ 56.104 and 56.105, the Food and Drug Administration may decide not to consider in support of an application for a research or marketing permit any data or information that has been derived from a clinical investigation that has not been approved by, and that was not subject to initial and continuing review by, an IRB meeting the requirements of this part.' Hence, adherence to these standards is not merely a regulatory obligation but a vital component of delivering safe and effective medical technologies. Additionally, the FDA's user experience engineering guidance is designed to be straightforward, promoting a risk-based documentation approach that expedites approval processes while ensuring patient safety.
The alignment between IEC 62366-1 and the FDA guidance improves the evaluation process, benefiting manufacturers of medical equipment. Key experts like Ana Criado, with her extensive experience in Regulatory Affairs and biomedical engineering, provide invaluable insights into navigating these complex requirements while ensuring a comprehensive understanding of project management and monitoring aspects.
Case Studies: Successful Integration of Human Factors in Medical Devices
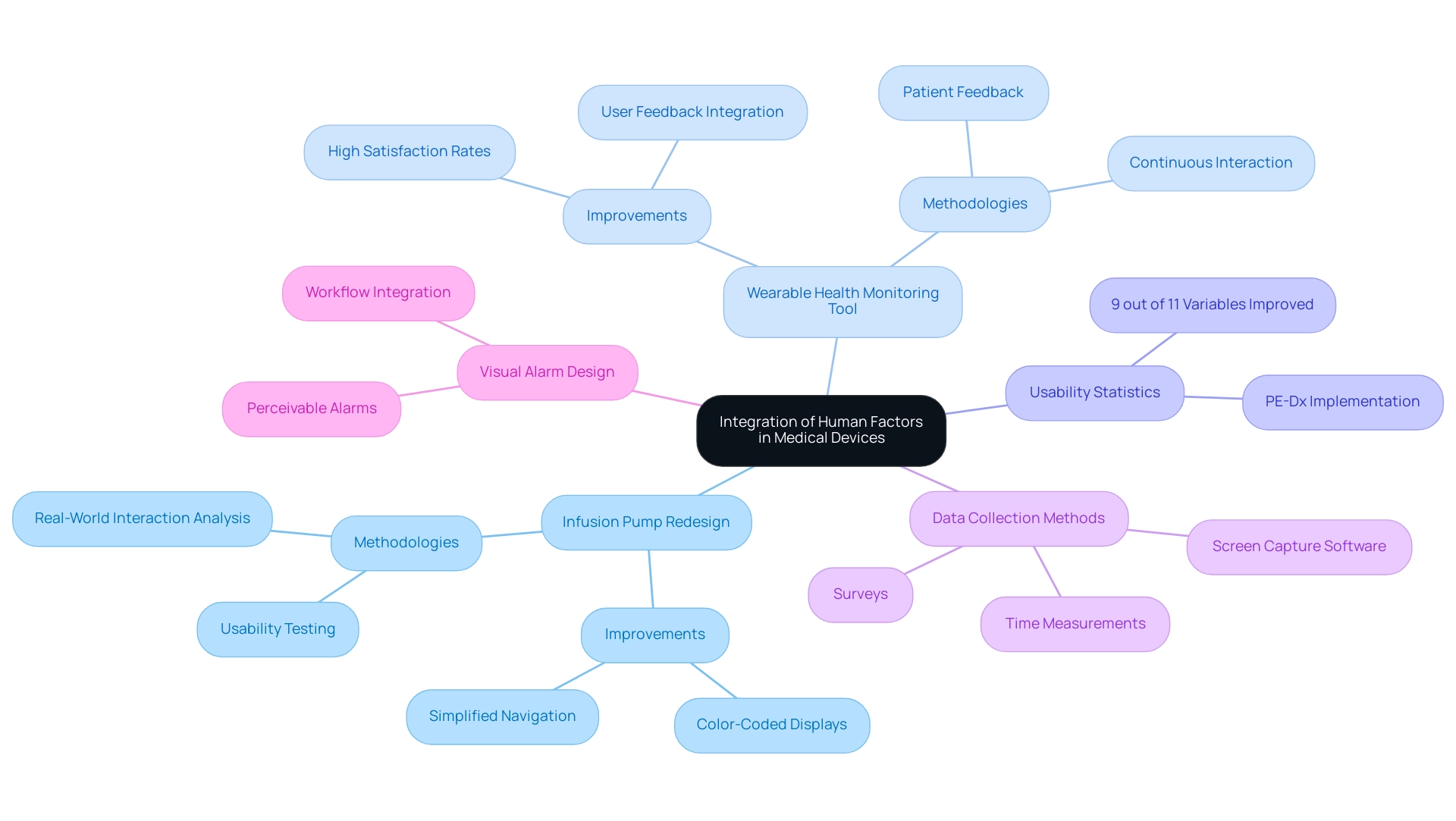
Several case studies showcase the effective implementation of human factors in medical device design, highlighting considerable improvements in user-friendliness and safety. One notable example is the redesign of an infusion pump by a prominent medical equipment manufacturer. This project included extensive usability testing with healthcare experts, which enabled the team to witness real-world interactions and identify crucial usability problems, particularly interface complexity and participant mistakes.
The redesign integrated user-friendly features such as color-coded displays and simplified navigation, resulting in a marked reduction in medication errors. Another compelling example is the creation of a wearable health monitoring tool that actively prioritized feedback from individuals throughout its development. By consistently interacting with patients and healthcare providers, the design team ensured the product effectively met genuine needs, which resulted in high satisfaction rates and enhanced adherence to monitoring protocols.
These cases emphasize the essential role of human factors in medical device design, which is crucial for creating instruments that are not only effective but also enhance user experience and safety within clinical settings. Recent studies have shown that 9 out of 11 user experience variables improved with the implementation of PE-Dx, further supporting the assertion that thoughtful integration of human factors in medical device design is crucial for successful medical device development. Additionally, as noted by Vincent Vandewalle, 'Alexandre Caron and Vincent Vandewalle contributed equally to the manuscript and should both be considered first author,' highlighting the collaborative efforts in advancing usability research.
Furthermore, a multifaceted approach to data collection and measurement was employed, utilizing screen capture software, surveys, and time measurements during task performance, allowing for a robust evaluation of interactions and decision-making processes.
Challenges and Future Directions in Human Factors Engineering
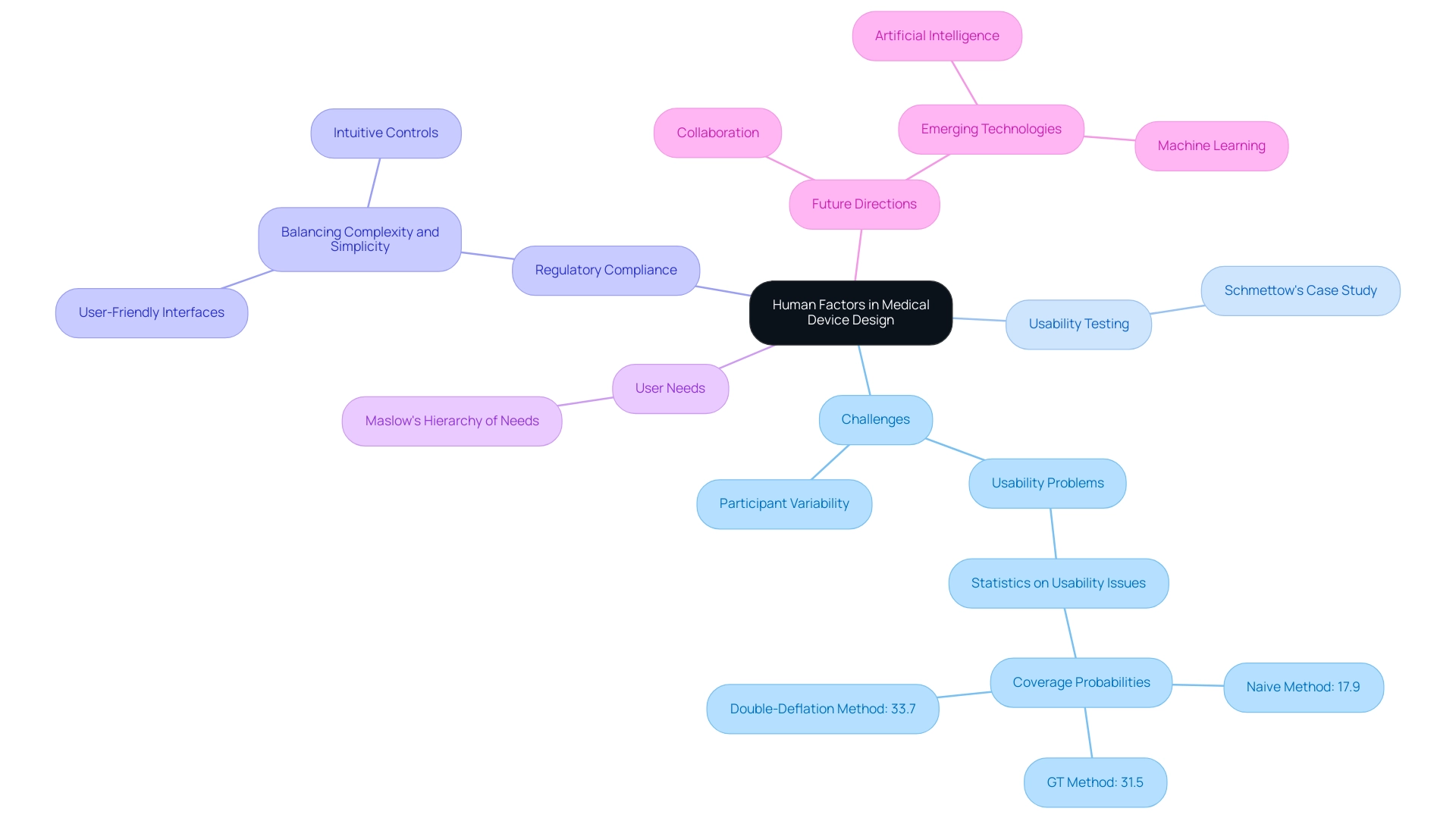
Incorporating human factors in medical device design into medical equipment development provides considerable advantages; nevertheless, various obstacles continue to persist. One of the most pressing issues is the variability in participant populations, which complicates the design process. Medical devices must cater to a diverse array of users, which highlights the importance of human factors in medical device design, including individuals with varying degrees of technical proficiency and physical abilities.
Schmettow’s usability testing of a medical infusion pump exemplifies the importance of adaptability, as it led to a redesign based on formative assessments that addressed usability challenges. Additionally, manufacturers often struggle to balance regulatory compliance with innovative design, facing constraints that can stifle creativity. A critical aspect of this balance is the need to consider human factors in medical device design, which includes friendly interfaces and intuitive controls that can simplify complex functionalities and enhance user experience.
Understanding Maslow's hierarchy of needs is essential for product designers to ensure that the medical devices meet users' basic needs effectively. Current statistics indicate that the average coverage probabilities for naive, GT, and double-deflation methods stand at 17.9%, 31.5%, and 33.7%, respectively, reflecting the need for robust testing practices. Furthermore, a case study comparing the estimated number of usability problems from five published studies using a matrix-based method against other methods highlights the effectiveness of different approaches in identifying usability challenges.
Looking toward the future, the landscape of human factors in medical device design will likely see enhanced collaboration among creators, engineers, and end-users. Embracing emerging technologies such as artificial intelligence and machine learning can provide deeper insights into individual behavior and preferences. This evolution is critical for developing adaptive designs that meet the needs of diverse user populations, ultimately leading to safer and more effective medical devices.
Conclusion
The exploration of human factors in medical device design reveals its critical role in enhancing patient safety and device efficacy. By understanding user interactions and incorporating principles of ergonomics and cognitive psychology, designers can create medical devices that not only function effectively but are also intuitive and user-friendly. The evidence from various case studies, such as the redesign of infusion pumps and wearable health devices, demonstrates that prioritizing user feedback during the design process leads to significant improvements in usability and safety, ultimately reducing the likelihood of user errors.
Furthermore, adherence to regulatory standards set forth by organizations like the FDA and ISO is essential in integrating human factors into device design. These regulations ensure that manufacturers conduct thorough usability testing and risk assessments, fostering a user-centered approach that benefits both stakeholders and patients alike. As highlighted by expert insights, the alignment of design practices with regulatory expectations not only streamlines compliance but also enhances overall patient outcomes.
Looking ahead, the challenges of variability in user populations and the need for innovative yet compliant designs remain pivotal concerns in the field. However, the future of human factors engineering appears promising, with potential advancements in technology and collaborative efforts among designers and end-users. Embracing these developments will be crucial in creating medical devices that are adaptable, safe, and effective, ultimately transforming the healthcare landscape for the better. The continued focus on human factors in medical device design is not merely a regulatory obligation but a vital strategy to improve the quality of care delivered to patients.
Frequently Asked Questions
What is the role of human factors in medical device design?
Human factors in medical device design focus on the interactions between individuals and systems, incorporating aspects like ergonomics, cognitive psychology, and user-focused design principles to enhance safety, effectiveness, and overall user experience.
How do human factors influence medical device usability?
A comprehensive understanding of human factors helps designers create products that minimize the likelihood of mistakes, thereby improving usability and patient outcomes.
What are some key practices for integrating human factors in medical device design?
Key practices include performing comprehensive interface evaluations, collecting ongoing participant feedback, and implementing clear labeling, intuitive interfaces, and ergonomic designs.
Why is participant feedback important in the development of medical devices?
Participant feedback is crucial for identifying real-world challenges early in the development process and guiding iterative enhancements to improve product performance.
What recent initiative highlighted functionality problems in medical device design?
The recent INFPUMP initiative revealed 107 functionality problems among 34 participants, emphasizing the need for early recognition of challenges in the development process.
How does the increase in telemedicine impact medical device research?
The rise of telemedicine allows for remote testing, which broadens participant recruitment in research studies, enhancing the diversity of feedback and enriching the development process.
What are some solutions that enhance user experience in medical devices?
Effective solutions include clear labeling, intuitive interfaces, and ergonomic designs, which significantly improve the experiences of healthcare providers and patients.
How do regulatory guidelines relate to human factors in medical device design?
Adhering to regulatory guidelines that emphasize human factors can streamline compliance during the approval process, facilitating faster market entry for innovative medical solutions.
What is the significance of post-market monitoring in medical device design?
Post-market monitoring involves gathering and analyzing functionality data to continuously enhance performance and user satisfaction, ensuring that products meet the real requirements of individuals.
What advice is given regarding feedback in medical device development?
It is advised to 'get feedback early and often,' as this approach is crucial for reducing medical equipment mistakes and ensuring that products satisfy the actual needs of users.




