Overview
Medical Device CROs (Contract Research Organizations) in Brazil are specialized entities that facilitate clinical trials and ensure compliance with local regulations, playing a crucial role in the development and commercialization of medical devices. The article emphasizes their importance by detailing the comprehensive services they provide, such as feasibility studies and project management, which are essential for navigating Brazil's complex regulatory landscape and enhancing patient outcomes in healthcare technology.
Introduction
In the rapidly evolving landscape of healthcare, Medical Device Contract Research Organizations (CROs) have emerged as pivotal players in the development and commercialization of innovative medical technologies. In Brazil, these specialized entities not only facilitate clinical trials but also navigate the complexities of stringent regulatory frameworks set forth by the Brazilian Health Regulatory Agency (ANVISA).
With the global medical device market witnessing a dynamic shift, highlighted by recent approvals from the US Food and Drug Administration, the role of CROs becomes increasingly critical. They ensure compliance, manage logistics, and uphold ethical standards throughout the clinical trial process, thereby enhancing patient outcomes and driving advancements in healthcare.
As Brazil's regulatory environment undergoes significant transformation, understanding the multifaceted challenges and opportunities that lie ahead for CROs is essential for stakeholders aiming to thrive in this competitive sector.
Defining Medical Device CROs: An Overview in the Brazilian Context
Healthcare Equipment Contract Research Organizations (CROs) are specialized entities that offer a broad range of services essential for the development and commercialization of healthcare products. In Brazil, Medical Device CRO Brazil organizations play a critical role in facilitating clinical trials by ensuring strict adherence to local regulations and effectively managing the logistics of research activities. The US Food and Drug Administration has approved nearly 105 medical devices since 2020, highlighting the dynamic nature of the global medical device market.
Given the complexities of the Brazilian healthcare environment, governed by comprehensive regulatory frameworks established by the Brazilian Health Regulatory Agency (ANVISA), a Medical Device CRO Brazil must navigate stringent requirements to ensure compliance. Their expertise encompasses a comprehensive clinical study management process that includes:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
Specifically, during setup, CROs meticulously prepare study protocols, secure necessary approvals, and coordinate with various stakeholders to ensure smooth execution.
Project management involves continuous monitoring of study progress, managing timelines, and addressing any issues that arise to keep the trial on track. A relevant example of successful healthcare technology commercialization is the Abbott MitraClip System, a minimally invasive instrument that treats mitral valve regurgitation, providing a less invasive alternative to open-heart surgery for certain patients. Furthermore, bioaccess® specializes in accelerated clinical study services for healthcare products across Latin America, concentrating on:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
This highlights the critical role of CROs in driving global health improvements through international collaboration and innovation in Medtech, ultimately contributing to advancements in healthcare and better patient outcomes. Furthermore, in January 2023, Charles River Laboratories announced the acquisition of SAMDI Tech, enhancing its portfolio of mass spectrometry solutions for biochemical activities, reflecting the ongoing evolution and investment in the CRO sector. To learn more about how we can assist you in advancing your healthcare equipment trials, SCHEDULE A MEETING with our team today.
Navigating Regulatory Changes: The Impact on Medical Device CROs in Brazil
The regulatory landscape for medical devices in Brazil, especially for Medical Device CRO Brazil, is undergoing significant transformation, driven by both domestic reforms and international standards. The recent enactment of Law No. 13.787/2018 introduces a series of critical changes that directly impact Contract Research Organizations.
This law mandates increased transparency, imposes stricter reporting obligations, and enhances oversight of clinical studies, compelling Medical Device CRO Brazil to reassess their operational frameworks. Notably, a precautionary mechanism can become a definitive penalty following an administrative proceeding, presenting additional operational challenges for Chief Risk Officers under the new regulations, such as the need to adapt processes quickly and ensure thorough compliance with evolving requirements.
To navigate these challenges effectively, Chief Risk Officers must invest in comprehensive clinical study management services provided by a Medical Device CRO Brazil, including:
- Feasibility and selection of research sites and principal investigators (PIs)
- Compliance reviews of study documents
- Study setup
- Import permits
- Robust project management and reporting systems
Compliance reviews are particularly crucial as they ensure that all study documents meet the stringent requirements set forth by the new law, reducing the risk of penalties.
Experts like Katherine Ruiz, specializing in regulatory affairs for medical devices and in vitro diagnostics in Colombia, alongside insights from Julio G. Martinez-Clark on commercialization, provide invaluable guidance in the context of Medical Device CRO Brazil in this evolving landscape.
The complexities of compliance are further illustrated by the case study on Cannabidiol (CBD) product manufacturing, where regulatory requirements necessitate federal authorization and adherence to specific practices, highlighting the intricate nature of the regulatory environment and the operational challenges contract research organizations must overcome to remain compliant.
As Brazil’s Minister of Health, Nísia Trindade, emphasizes,
the modernization of regulatory processes is essential for advancing medical innovation in Brazil.
This sentiment reflects the pressing necessity for the Medical Device CRO Brazil to align with changing regulations to better assist their clients and ensure successful study results. Comprehending these recent developments is not merely beneficial; it is imperative for clinical research organizations aiming to thrive in this dynamic regulatory environment.
The Role of CROs in Clinical Trials for Medical Devices
Medical Device CRO Brazil plays an essential role as a participant in the management of clinical studies for medical devices, serving as a crucial intermediary between device manufacturers and regulatory authorities. As a Medical Device CRO Brazil, their responsibilities encompass comprehensive planning and execution of clinical studies, including:
- Crafting detailed study protocols
- Recruiting diverse participant populations
- Meticulously collecting and analyzing [data
Contract research organizations](https://clinregs.niaid.nih.gov/country/brazil) also prepare regulatory submissions to ensure compliance with applicable laws and standards.
As highlighted by ResNo466, obtaining informed consent is critical, particularly for minors or those unable to consent independently, necessitating the use of assent forms. In regions like Uruguay, Cuba, and Paraguay, where high incidence and mortality rates are prevalent but clinical trials are scarce, the involvement of a Medical Device CRO Brazil is crucial to enhance clinical research efforts. bioaccess® stands out as a vetted Medical Device CRO Brazil and consulting partner for U.S. device companies in Colombia, leveraging over 20 years of experience in Medtech to offer expertise in managing various studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
Furthermore, clinical research organizations play a crucial role in ensuring data protection and compliance with regulations, as illustrated by the case study on Data Protection Principles Under LGPD, emphasizing the principles of purpose, adequacy, necessity, and transparency in processing personal data. By utilizing their specialized expertise and operational abilities, the Medical Device CRO Brazil not only enables the effective execution of studies but also maintains ethical standards, thus speeding up the advancement of groundbreaking medical technologies while greatly improving the quality of data generated. bioaccess® utilizes a personalized method designed for the distinct requirements of each study, ensuring that particular challenges encountered in Latin America are addressed.
Consequently, contract research organizations play a pivotal role in improving patient outcomes, an increasingly important consideration in global research agendas, particularly in light of the underrepresentation of diverse populations in clinical trials across regions like Latin America and the Caribbean.
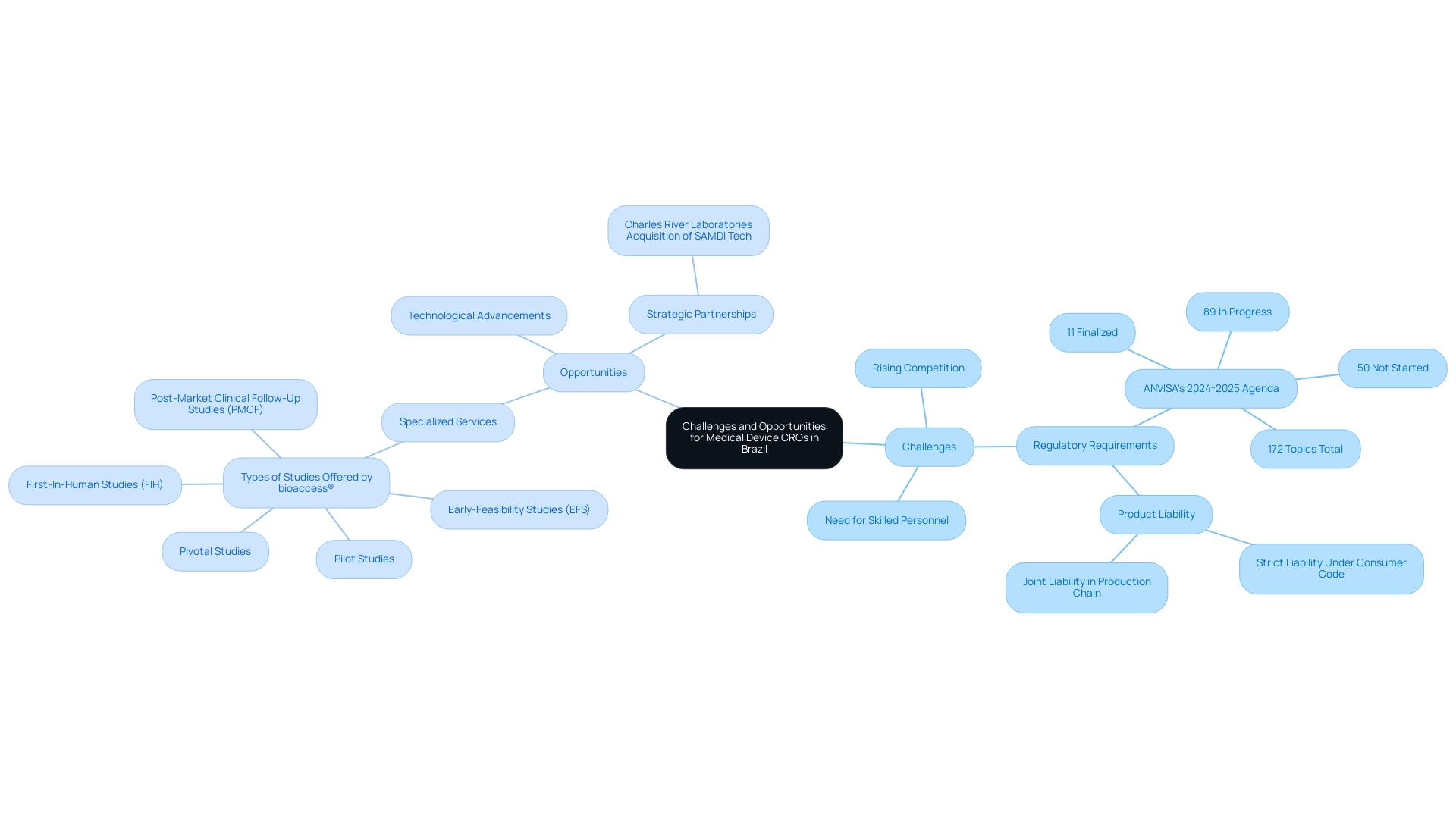
Challenges and Opportunities for Medical Device CROs in Brazil
Medical Device CRO Brazil encounters a multifaceted set of challenges, including stringent regulatory requirements, rising competition, and a critical need for skilled personnel. As outlined in ANVISA's 2024-2025 regulatory agenda, which includes 172 topics—89 of which are currently in progress—these organizations must adeptly navigate the complexities of regulatory compliance while ensuring the safety and effectiveness of healthcare products post-market introduction. The Brazilian Consumer Code imposes strict liability on companies for damages caused by defective products, highlighting the necessity for thorough investigatory records and compliance throughout the production chain, which directly impacts CROs' operational strategies.
Furthermore, bioaccess® stands out with its accelerated clinical study services for healthcare products, showcasing a deep expertise in managing various study types, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
With over 20 years of experience in the Medtech industry, the bioaccess® team is well-equipped to address the increasing complexity of clinical trials and meet the escalating demand for innovative medical devices. Despite the challenges, there are significant opportunities for clinical research organizations to carve out a competitive advantage.
By concentrating on specialized services, utilizing technological advancements, and creating strategic partnerships, contract research organizations can establish themselves as leaders within niche markets or emerging technologies. For instance, Charles River Laboratories illustrated this with its acquisition of SAMDI Tech to enhance its mass spectrometry solutions, showcasing how strategic growth initiatives are vital for contract research organizations to effectively address the evolving needs of the healthcare sector. Ultimately, by leveraging these opportunities, Medical Device CRO Brazil can not only overcome existing challenges but also contribute significantly to advancements in healthcare technology, job creation, and improved patient care within local economies.
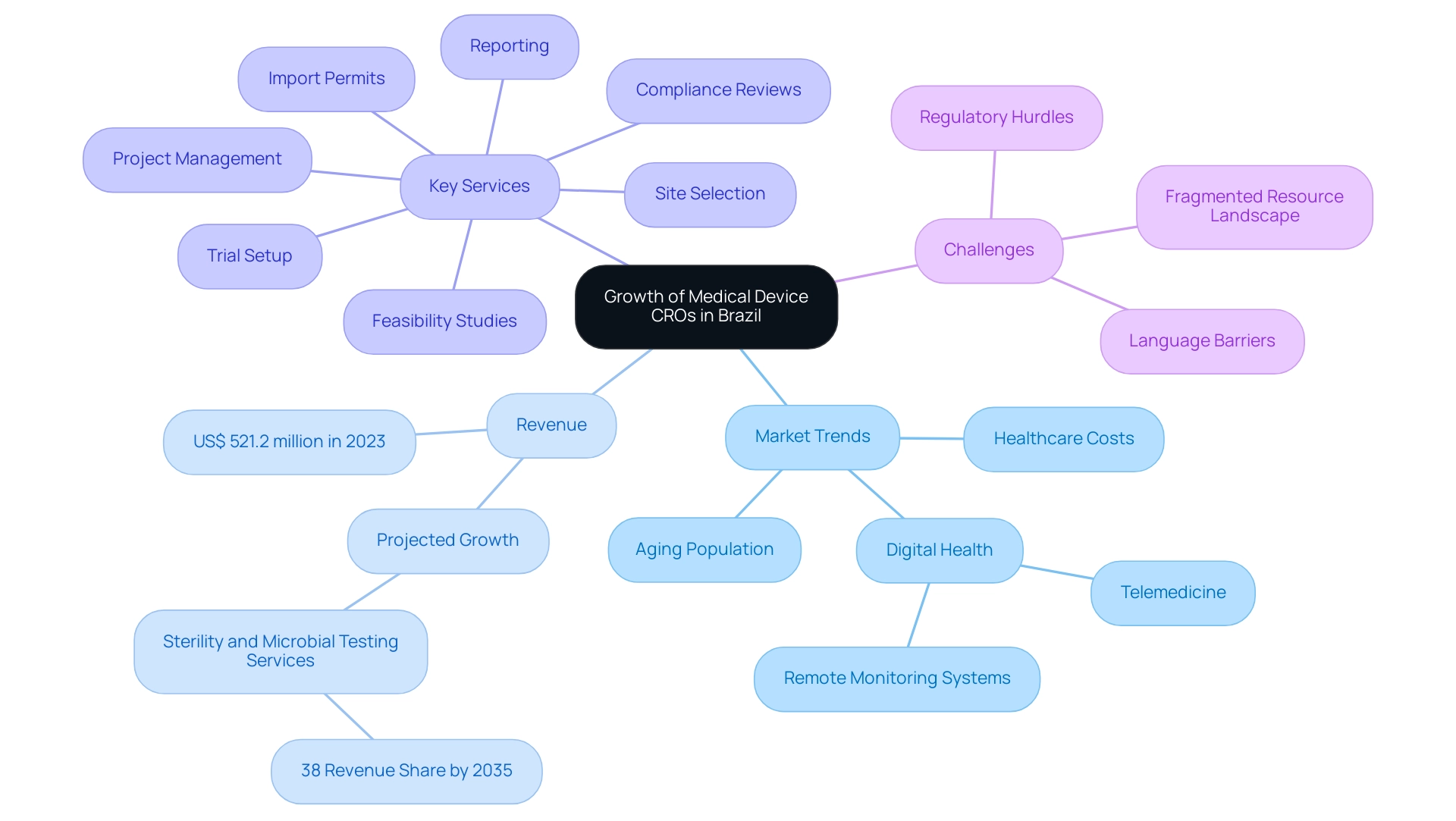
Future Trends: The Growth of Medical Device CROs in Brazil
The outlook for the Medical Device CRO Brazil is exceptionally promising, driven by several pivotal trends. The demand for innovative healthcare technologies is escalating, fueled in part by the nation's aging population and increasing healthcare costs. These factors are anticipated to significantly boost clinical research activities across the region.
Notably, the revenue for the medical device contract research organization market in Latin America reached approximately US$ 521.2 million in 2023, reflecting the sector's robust growth potential. Additionally, sterility and microbial testing services are expected to hold the maximum share of preclinical services, capturing 38% of revenue share by 2035, highlighting a crucial aspect of market dynamics. Furthermore, advancements in digital health technologies, including telemedicine and remote monitoring systems, are expected to enhance clinical study efficiency.
Experts predict that as more Medical Device CRO Brazil companies, like bioaccess®, enter the Brazilian market, this influx will drive innovation and quality standards, essential for the successful introduction of new technologies. As emphasized in the collaboration between Greenlight Guru and bioaccess™, efforts to bridge the gaps in clinical research and innovation will be key to accelerating Medtech advancements, as exemplified by PAVmed's First-In-Human Study in Colombia. However, US Medtech companies face specific challenges, including regulatory hurdles, language barriers, and a fragmented resource landscape, which can complicate their operations in Latin America.
Additionally, the clinical research sector in Latin America has experienced substantial investment, notably with the pharmaceutical industry pouring $980 million into Brazil, Argentina, and Mexico. This investment has triggered an impressive 138% increase in healthcare equipment assessments between 2017 and 2020, fueled by high patient retention rates and robust patient-physician relationships. bioaccess® offers comprehensive clinical trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
ensuring that new technologies are introduced with the utmost safety and efficacy.
As Medical Device CRO Brazil adapts to these shifting dynamics, it will play an indispensable role in the evolution of medical device development in Brazil, ultimately enhancing patient care.
Conclusion
The significance of Medical Device Contract Research Organizations (CROs) in Brazil's healthcare landscape cannot be overstated. As facilitators of clinical trials, these organizations navigate a complex regulatory environment while driving innovation and ensuring compliance with the stringent regulations set forth by the Brazilian Health Regulatory Agency (ANVISA). Their expertise encompasses a variety of critical functions, from feasibility studies to project management, all aimed at enhancing patient outcomes and accelerating the introduction of new medical technologies.
The ongoing regulatory transformations present both challenges and opportunities for CROs. As new laws and guidelines emerge, organizations must adapt their operational frameworks to maintain compliance and uphold ethical standards. This adaptability is crucial not only for meeting regulatory demands but also for positioning themselves as leaders in the competitive medical device market. By investing in robust clinical trial management and leveraging their specialized knowledge, CROs can overcome obstacles and contribute to the advancement of healthcare in Brazil.
Looking ahead, the growth potential for CROs in Brazil is promising, fueled by increasing demand for innovative medical technologies and the expansion of clinical research activities. The influx of investment in the region, coupled with advancements in digital health technologies, will likely enhance the efficiency of clinical trials and drive further innovation. As CROs continue to adapt to these evolving dynamics, their role will be pivotal in shaping the future of medical device development, ultimately leading to improved patient care and health outcomes across the country.
Frequently Asked Questions
What are Healthcare Equipment Contract Research Organizations (CROs)?
Healthcare Equipment CROs are specialized entities that provide a range of services essential for the development and commercialization of healthcare products, including facilitating clinical trials and ensuring compliance with regulations.
What role do Medical Device CROs play in Brazil?
Medical Device CROs in Brazil facilitate clinical trials by ensuring adherence to local regulations set by the Brazilian Health Regulatory Agency (ANVISA) and managing the logistics of research activities.
What services do Medical Device CROs in Brazil offer?
They offer services including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting.
How do CROs ensure smooth execution during study setup?
During setup, CROs prepare study protocols, secure necessary approvals, and coordinate with various stakeholders to ensure smooth execution of clinical trials.
What is involved in project management by CROs?
Project management involves continuous monitoring of study progress, managing timelines, and addressing any issues that arise to keep the trial on track.
Can you provide an example of successful healthcare technology commercialization?
The Abbott MitraClip System is an example of successful commercialization; it is a minimally invasive instrument that treats mitral valve regurgitation, offering a less invasive alternative to open-heart surgery.
What specific studies does bioaccess® focus on in Latin America?
Bioaccess® specializes in accelerated clinical study services, focusing on Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
What recent changes have impacted Medical Device CROs in Brazil?
The enactment of Law No. 13.787/2018 has introduced critical changes affecting Medical Device CROs, including increased transparency, stricter reporting obligations, and enhanced oversight of clinical studies.
What challenges do Chief Risk Officers face under the new regulations?
Chief Risk Officers must adapt processes quickly to ensure compliance with evolving requirements, as non-compliance can lead to penalties following administrative proceedings.
Why are compliance reviews important for CROs?
Compliance reviews ensure that all study documents meet the stringent requirements set by new regulations, reducing the risk of penalties for the organization.
Who provides guidance on regulatory affairs for Medical Device CROs in Brazil?
Experts such as Katherine Ruiz, who specializes in regulatory affairs for medical devices, and Julio G. Martinez-Clark, who provides insights on commercialization, offer invaluable guidance in this evolving landscape.
What is the significance of modernizing regulatory processes in Brazil?
Modernizing regulatory processes is essential for advancing medical innovation in Brazil, as emphasized by the Minister of Health, Nísia Trindade, highlighting the need for CROs to align with changing regulations for successful study outcomes.




