Introduction
The role of Medical Device Contract Research Organizations (CROs) has become increasingly vital in the rapidly evolving MedTech landscape. These entities serve as essential partners in the development and commercialization of medical devices, offering a comprehensive suite of services that streamline clinical research processes. As the industry anticipates a robust growth trajectory, the challenges faced by US MedTech companies, particularly in Latin America, highlight the necessity for effective collaboration and innovative solutions.
This article delves into the multifaceted functions of Medical Device CROs, the regulatory complexities they navigate, and the emerging trends that are shaping the future of clinical research. By examining the integral role of CROs, the discussion underscores their impact on advancing medical science and improving patient outcomes in an increasingly competitive environment.
Defining Medical Device CROs: Key Concepts and Importance
A Medical Device CRO is an essential entity in the MedTech sector, providing a wide array of specialized services to facilitate the development and commercialization of medical devices. These organizations are crucial in medical research, expertly overseeing various phases of studies, from design and regulatory submissions to patient recruitment and data management. The significance of Medical Device CROs is highlighted by the industry's projected growth rate of 5.6% annually, emphasizing their vital role in accelerating the time-to-market for innovative solutions.
However, US Medtech firms encounter substantial difficulties in Latin America, including regulatory obstacles, language barriers, and resource fragmentation, which complicate cooperation with local hospitals and research locations. Significantly, collaborations like Greenlight Guru's alliance with bioaccess™ seek to improve research efficiencies in Latin America, directly tackling these challenges. Additionally, the successful first in-human trial by PAVmed in Colombia exemplifies the tangible outcomes of these collaborations.
As observed by Tulio Sanquiz, 'The Clinical Research Associate oversees the advancement of research studies at investigative locations or from a distance, ensuring that experiments are carried out, documented, and reported following the protocol.' This underscores the responsibility of the Medical Device CRO in maintaining compliance with stringent regulatory requirements, allowing medical device manufacturers to focus on their core competencies. By collaborating with a Medical Device CRO, manufacturers can navigate the complexities of trials more efficiently, ultimately contributing to the advancement of medical science and the development of groundbreaking therapies.
Furthermore, GlobalCare Clinical Trials’ partnership with bioaccess™ has led to over a 50% reduction in recruitment time and a retention rate exceeding 95%, demonstrating the effectiveness of such collaborations. The need for a solution-focused approach to bridge gaps in medical research and innovation is critical, as highlighted by these successful partnerships. Careers in medical research provide numerous opportunities for professionals to contribute to medical science, thereby enhancing the workforce within this critical sector.
An illustrative case study, titled 'Why Using Coas and DHTs Together is the Future of Research,' explores innovative approaches to research methodologies, showcasing how Medical Device CROs can effectively integrate Clinical Outcome Assessments with Digital Health Technologies.
Comprehensive Services Offered by Medical Device CROs in Clinical Research
Medical Device CROs offer a comprehensive array of services specifically designed to address the unique challenges of clinical research within the MedTech sector. Their expertise spans several critical areas:
-
Regulatory Affairs: Medical Device contract research organizations assist clients in navigating complex regulatory frameworks, ensuring successful submissions to key agencies such as the FDA and EMA.
This support is essential, especially considering that the FDA requires post-market safety and effectiveness evaluations, which unfortunately are not always carried out. This gap underscores the challenges CROs encounter in ensuring that post-market evaluations are conducted to uphold patient safety and product efficacy.
-
Research Study Management: These organizations oversee the complete lifecycle of research studies, from detailed planning to execution and oversight, thereby ensuring adherence to established protocols and regulatory standards. They also provide extensive research study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting, which are essential for effective study outcomes.
-
Site Management: By identifying, qualifying, and managing clinical research locations, contract research organizations optimize patient recruitment and enhance data collection processes, addressing common recruitment issues faced by medical device startups.
-
Data Management and Biostatistics: They provide advanced data analysis and management services that support the integrity and reliability of research data, which is crucial in fulfilling regulatory requirements.
-
Quality Assurance: Implementing rigorous quality control measures, Medical Device Contract Research Organizations maintain compliance with Good Clinical Practice (GCP) and other regulatory guidelines, ensuring high standards are met throughout the research process.
-
Document Review: Clinical research organizations offer comprehensive evaluation and feedback on research documents to ensure adherence to country-specific regulations, which is essential for successful project initiation.
By delivering these pivotal services, Medical Device contract research organizations significantly enhance the efficiency and effectiveness of clinical research, directly contributing to the progression of medical technologies and ultimately resulting in improved patient outcomes. Bioaccess®, with its expertise in early-feasibility, first-in-human, pilot, pivotal, and post-market follow-up studies, exemplifies the critical role contract research organizations play in navigating the complexities of medical device trials in Latin America.
As highlighted by industry experts, despite receiving priority review, fast-track status, and accelerated approval, the drug showed minimal effectiveness against a surrogate endpoint. This underscores the vital role clinical research organizations play in ensuring that expedited approvals are backed by substantial evidence. Furthermore, ethical concerns regarding the adequacy of evidentiary standards in FDA approvals emphasize the necessity for contract research organizations to advocate for more rigorous requirements and better education for physicians on communicating uncertainty.
To explore how we can assist you further, BOOK A MEETING today.
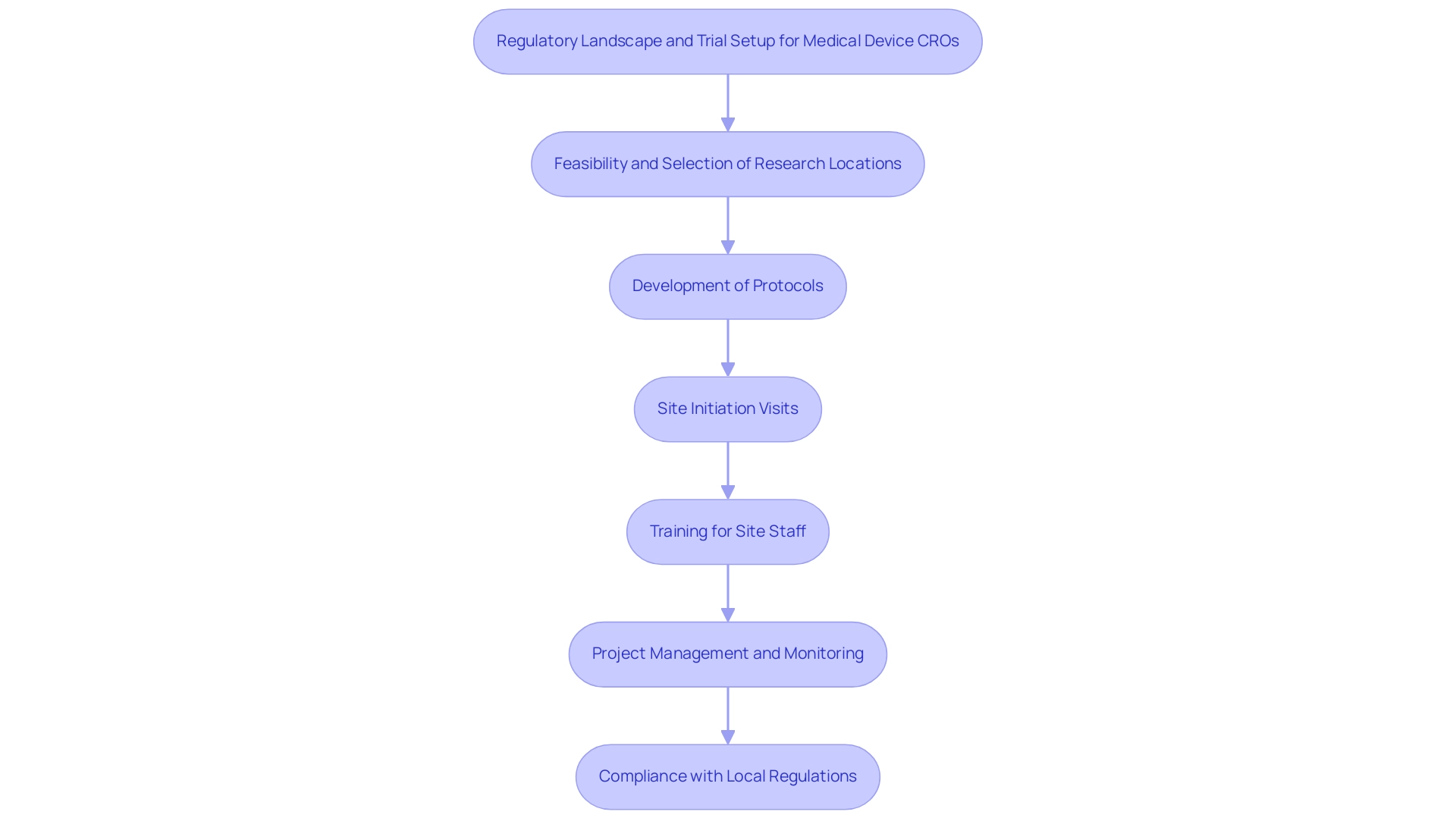
The Regulatory Landscape for Medical Device CROs
Navigating the regulatory landscape for Medical Device CRO presents a complex challenge that varies significantly across regions. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the approval process for medical devices, mandating that CROs adhere to stringent guidelines throughout clinical studies. A notable aspect of this process is the recent guidance issued in July 2017 regarding the Institutional Review Board (IRB) waiver or alteration of informed consent for minimal risk research, highlighting the evolving nature of FDA regulations and the agency's commitment to ethical oversight.
Furthermore, the FDA emphasizes that ECs and investigators should carefully consider whether the inclusion in research of individuals who lack consent capacity is ethically appropriate and scientifically necessary.
The extensive service capabilities of a Medical Device CRO encompass:
- Feasibility and selection of research locations and principal investigators (PIs)
- Detailed reviews and feedback on project documents to ensure adherence to country-specific requirements
- Careful setup and approval processes involving both ethics committees and health ministries
The trial setup process encompasses:
- Development of protocols
- Site initiation visits
- Training for site staff to ensure adherence to regulatory standards
The import permit and nationalization of investigational devices are also critical components, along with dedicated project management and monitoring, which encompass:
- Reporting on project status
- Inventory
- Adverse events—both serious and non-serious
It is essential for project management to include compliance with local regulations, as this can significantly impact the timeline and success of the study.
In Europe, the Medical Device Regulation (MDR) imposes comprehensive requirements regarding evaluations, reinforcing the need for rigorous compliance. The RevComRule allows for the use of identifiable information or biospecimens if deemed necessary by the institutional ethics committee, further complicating the regulatory landscape. Ensuring compliance with these evolving regulations is crucial, as non-adherence can lead to significant delays, financial penalties, and reputational damage.
Medical Device CROs must remain vigilant and informed about the evolving regulatory landscape to ensure that all studies comply with the latest standards. Katherine Ruiz, a Regulatory Affairs expert in medical devices and in vitro diagnostics, has extensive experience advising foreign manufacturers on obtaining market clearance in Colombia. Her background, including her tenure at Colombia's regulatory agency INVIMA, equips her with invaluable insights into navigating these complexities.
The United States' founding membership in the International Council for Harmonization (ICH) exemplifies the global push towards standardization in research practices. The adoption of ICH guidelines improves the quality and consistency of medical studies, both nationally and globally, ultimately protecting patient safety and preserving data integrity.
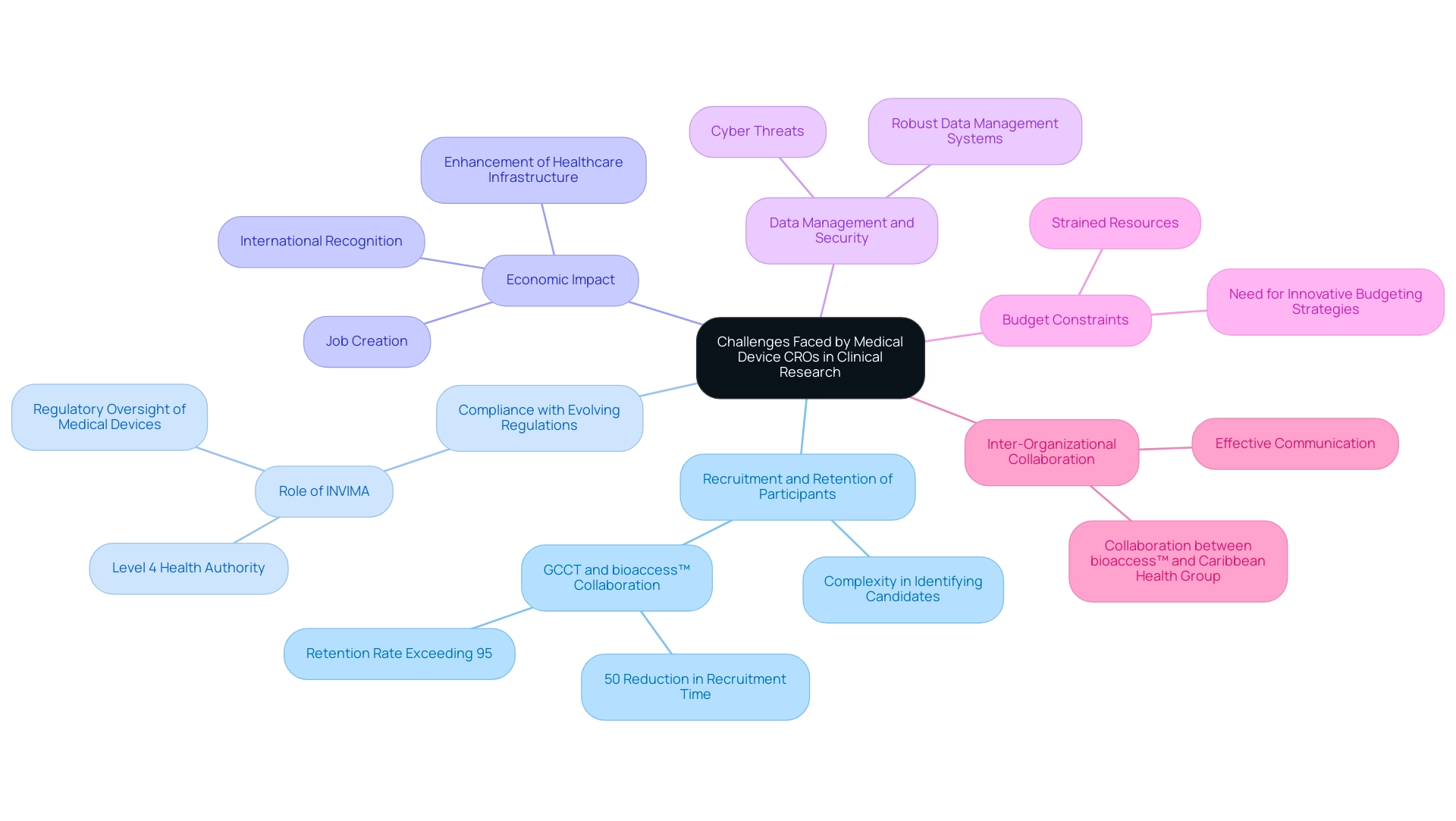
Challenges Faced by Medical Device CROs in Clinical Research
The research landscape for navigating challenges in the Medical Device CRO sector requires strategic solutions. Key hurdles include:
-
Recruitment and Retention of Participants: Identifying and retaining suitable candidates for clinical studies is increasingly complex, particularly when dealing with specialized medical devices that target specific patient populations.
Statistics indicate that recruitment difficulties can lead to delays in project timelines and increased costs. A notable example is the partnership between GlobalCare Clinical Trials (GCCT) and bioaccess™, which has led to over a 50% reduction in recruitment time and a retention rate exceeding 95%. This illustrates how effective collaboration can enhance recruitment strategies in overcoming challenges.
-
Compliance with Evolving Regulations: The regulatory environment is continually shifting, demanding that CROs remain vigilant and adaptable. This not only strains resources but also necessitates ongoing training and updates to operational protocols to ensure compliance.
The Colombia National Food and Drug Surveillance Institute (INVIMA) plays a crucial role in this process, overseeing medical device regulations and ensuring adherence to high standards, as recognized by the Pan American Health Organization/World Health Organization's classification of INVIMA as a Level 4 health authority. INVIMA's specific functions involve overseeing and regulating medical devices, which is crucial for ensuring adherence in trials.
-
Economic Impact: Clinical trials significantly affect local economies, generating employment, fostering economic development, and enhancing healthcare infrastructure. These studies not only enhance the local workforce but also contribute to international recognition of the region as a hub for clinical research.
-
Data Management and Security: With cyber threats on the rise, ensuring the integrity and confidentiality of trial data is paramount. Medical Device CROs must implement robust data management systems to protect sensitive information, adhering to the highest standards of data security.
-
Budget Constraints: Many CROs operate under stringent budget limitations, which can lead to compromises in quality if resources are not allocated efficiently. This challenge emphasizes the need for innovative budgeting strategies that maximize impact while adhering to financial constraints.
-
Inter-Organizational Collaboration: Effective communication and cooperation among various stakeholders—including sponsors, regulatory bodies, and research sites—are crucial for the success of studies. However, achieving seamless collaboration can be challenging due to differing priorities and operational practices. The collaboration between bioaccess™ and Caribbean Health Group exemplifies how strategic partnerships can position Barranquilla as a leading destination for medical research in Latin America, showcasing the benefits of strong inter-organizational relationships.
By systematically identifying and addressing these challenges, strategies devised by Medical Device CROs can enhance operational efficiency and ultimately lead to improved research outcomes. Integrating these strategies, along with insights gained from successful partnerships, can greatly improve participant recruitment and retention initiatives in research studies. Moreover, thorough research management services—including feasibility studies, site selection, and project oversight—are vital for navigating these challenges effectively.
Future Trends in Medical Device CROs and Clinical Research
The landscape of clinical research is in a state of continuous evolution, compelling Medical Device CROs to adapt to emerging trends to maintain their competitive edge. Key trends shaping this transformation include:
-
Increased Use of Technology: The integration of digital tools, such as electronic data capture (EDC) and telemedicine, is not only streamlining data collection but also significantly enhancing patient engagement.
This technological advancement is crucial as it increases data accuracy and facilitates real-time monitoring.
-
Focus on Patient-Centric Approaches: A marked shift towards designing studies that prioritize patient experiences and outcomes has emerged.
This focus leads to more meaningful data gathering and reflects a commitment to addressing patient needs, ultimately enhancing relevance and effectiveness.
-
Regulatory Innovation: Regulatory bodies are increasingly embracing adaptive trial designs and real-world evidence, which expedite approvals and enhance the agility of CRO methodologies.
However, US Medtech companies still face significant regulatory hurdles and language barriers when conducting studies in Latin America, complicating their efforts to navigate local regulations and communicate effectively with stakeholders.
-
Collaboration and Partnerships: Forming strategic alliances, such as the recent collaboration between Greenlight Guru and bioaccess™, allows contract research organizations to leverage specialized expertise and resources, fostering innovation and improving research outcomes.
This partnership aims to expedite early-stage medical studies in Latin America, tackling the specific challenges encountered by Medtech companies in the region, including the need for local expertise and assistance.
-
Comprehensive Research Study Management Services: Contract research organizations are concentrating on complete oversight of research studies, including feasibility evaluations, site selection, compliance reviews, study setup, import permits, project management, and reporting.
Such comprehensive services are vital for navigating the complexities of research trials in Latin America.
-
Sustainability Practices: As worldwide environmental issues become more significant, Contract Research Organizations are investigating sustainable methods within research, aiming to lessen their carbon footprint while positively impacting the global health landscape.
For instance, the case analysis of Pit Vidura illustrates an innovative solution improving access to safe sanitation in Kigali, Rwanda, demonstrating how sustainability practices can lead to positive public health outcomes.
By proactively engaging with these trends, Medical Device CROs can solidify their position as leaders in the research domain, driving advancements in medical technologies and enhancing patient care. Furthermore, the impact of clinical studies on local economies is significant, contributing to job creation, economic growth, and healthcare improvement.
Initiatives like CBIV’s ambitious target to save 500,000 lives and improve the health of 10 million underserved women and children in emerging markets exemplify the positive impact that aligned research efforts can have on global health equity.
As Maria Tegborg from the Swedish International Development Cooperation Agency (Sida) aptly notes, 'Too little attention is being paid to women’s and children’s health in low- and middle-income countries and there are too few women venture capitalists,' underscoring the urgent need for focused investment in these areas.
Conclusion
The role of Medical Device Contract Research Organizations (CROs) is undeniably crucial in navigating the complexities of clinical research within the MedTech sector. These organizations not only streamline the development and commercialization of innovative medical devices but also tackle significant challenges such as regulatory compliance, participant recruitment, and data management. By providing a comprehensive suite of services, CROs enhance the efficiency of clinical trials, thereby accelerating the time-to-market for essential medical solutions.
As the MedTech landscape continues to evolve, it is imperative for CROs to adapt to emerging trends, including the increased use of technology, patient-centric trial designs, and strategic collaborations. These trends not only improve research outcomes but also align with the broader goal of enhancing patient care and advancing medical science. The successful partnerships and innovative practices highlighted throughout the discussion exemplify the potential for CROs to overcome regional challenges and contribute positively to local economies.
In conclusion, the importance of Medical Device CROs cannot be overstated. They serve as vital partners in the advancement of medical technologies, ultimately leading to improved patient outcomes and a more effective healthcare system. As the industry anticipates continued growth, embracing collaboration and innovation will be key to addressing the evolving needs of the MedTech sector and ensuring that clinical research remains at the forefront of medical advancements.
Frequently Asked Questions
What is a Medical Device CRO?
A Medical Device CRO (Contract Research Organization) is an essential entity in the MedTech sector that provides specialized services to facilitate the development and commercialization of medical devices. They oversee various phases of studies, including design, regulatory submissions, patient recruitment, and data management.
Why are Medical Device CROs significant in the industry?
Medical Device CROs play a crucial role in medical research and are vital for accelerating the time-to-market for innovative solutions. The industry's projected growth rate of 5.6% annually underscores their importance.
What challenges do US Medtech firms face in Latin America?
US Medtech firms encounter regulatory obstacles, language barriers, and resource fragmentation, complicating cooperation with local hospitals and research locations.
How do collaborations improve research efficiencies in Latin America?
Collaborations, such as Greenlight Guru's alliance with bioaccess™, aim to tackle challenges faced by Medtech firms in Latin America, improving research efficiencies and facilitating better outcomes.
What are the key services provided by Medical Device CROs?
Medical Device CROs offer services in several critical areas, including: 1. Regulatory Affairs 2. Research Study Management 3. Site Management 4. Data Management and Biostatistics 5. Quality Assurance 6. Document Review
How do Medical Device CROs assist with regulatory compliance?
They help navigate complex regulatory frameworks, ensuring successful submissions to agencies like the FDA and EMA, and maintaining compliance with stringent regulatory requirements throughout the research process.
What impact do Medical Device CROs have on clinical trial participant recruitment?
Medical Device CROs optimize patient recruitment and enhance data collection processes, addressing common recruitment issues faced by medical device startups, leading to improved recruitment times and retention rates.
How do Medical Device CROs ensure data integrity and security?
They implement robust data management systems to protect sensitive information and adhere to high standards of data security, which is crucial in fulfilling regulatory requirements.
What trends are shaping the landscape of clinical research for Medical Device CROs?
Key trends include: 1. Increased use of technology 2. Focus on patient-centric approaches 3. Regulatory innovation 4. Collaboration and partnerships 5. Comprehensive research study management services 6. Sustainability practices
How do Medical Device CROs contribute to local economies?
Clinical trials significantly impact local economies by generating employment, fostering economic development, and enhancing healthcare infrastructure, contributing to international recognition of the region as a hub for clinical research.




