Overview
Medtech CRO services refer to the comprehensive outsourced research functions critical for the development and commercialization of medical technologies, including feasibility evaluations, compliance management, and clinical study oversight. The article emphasizes their importance in ensuring regulatory compliance and operational efficiency, as evidenced by successful partnerships that have significantly reduced clinical trial timelines and improved patient recruitment rates.
Introduction
The landscape of medical technology is evolving rapidly, driven by the increasing complexity of clinical trials and the stringent regulatory environment that governs them. Medtech Contract Research Organizations (CROs) have emerged as indispensable partners in this realm, providing a range of services that facilitate the development and commercialization of innovative medical devices and therapies.
From regulatory compliance and clinical trial management to the integration of advanced technologies such as AI and data analytics, CROs are at the forefront of ensuring that medical innovations not only meet rigorous standards but also reach the market efficiently.
As the industry grapples with emerging trends and challenges, understanding the pivotal role of Medtech CROs becomes essential for stakeholders aiming to navigate this dynamic landscape successfully.
Defining Medtech CRO Services: An Overview
The comprehensive array of outsourced research functions vital for the development and commercialization of medical technologies is covered by Medtech CRO Services. These services encompass:
- Feasibility evaluations
- Investigator selection
- Compliance with regulations
- Project management for studies
- Comprehensive reporting
All are vital for assisting medical device producers and pharmaceutical firms. A critical component of these services is the review and feedback on study documents to ensure compliance with country requirements, as well as obtaining import permits and nationalizing investigational devices.
By serving as essential collaborators in the research process, Medtech organizations support the effective advancement of medical studies while ensuring compliance with strict guidelines. Notably, GlobalCare Clinical Trials' partnership with bioaccess™ has led to an impressive reduction of over 50% in clinical trial subject recruitment time and a retention rate exceeding 95%, showcasing the impact of effective Medtech CRO Services in real-world scenarios. These outcomes can be directly linked to the extensive assistance offered by Medtech CRO Services, which includes project management and compliance advice.
Similarly, IDx Technologies is leveraging partnerships to enhance data-licensing collaboration in Latin America, which is crucial for advancing AI-driven disease detection in ophthalmology. According to industry insights, 47% of professionals believe that enhanced quality management is pivotal for driving innovation within the sector. As Jim Welch, EY Global MedTech Leader, states, 'Quality management is not just a regulatory requirement; it's a catalyst for innovation in MedTech.'
Furthermore, with the recent allocation of $2.6 billion to MedTech OEMs in the USA, particularly focusing on smaller startups, Medtech CRO Services are well-positioned for contract research organizations to leverage their expertise in navigating these opportunities. The case study titled 'Strategic Insights for MedTech Service Providers' highlights that understanding funding distribution across therapeutic areas can help contract research organizations better target their efforts and align with market opportunities, ultimately contributing to improved patient care.
Key Services Provided by Medtech CROs
Medtech Contract Research Entities play a crucial role in the development and adherence to regulations of medical technologies by providing a range of essential services, including:
- Compliance Affairs: This service is essential for helping clients in maneuvering through the complex oversight landscape, ensuring conformity with both local and international laws. As stated by Regulatory Affairs specialist Julio G. Martinez-Clark, furthermore, contract research organizations often have established relationships with regulators and a profound understanding of ever-changing regulations, which is invaluable for navigating the complex and challenging pathways from concept to market.
- Comprehensive Clinical Study Management: Medtech CRO Services oversee the meticulous planning, execution, and monitoring of clinical studies, including feasibility assessments and site selection. Their expertise ensures that all stages of the process adhere to established protocols and timelines, thereby optimizing operational efficiency. This includes the review and feedback on study documents to comply with country requirements, as well as trial setup, start-up, and approval processes involving ethics committees and health ministries. Specifically, clinical research organizations facilitate the preparation and submission of necessary documentation to ethics committees to secure approvals, ensuring that all ethical considerations are met before the commencement of the trial.
- Import Permits and Nationalization of Investigational Devices: Clinical research organizations assist in securing necessary import permits and ensuring the nationalization of investigational devices, facilitating smoother transitions into different markets.
- Data Management: These organizations excel in collecting, analyzing, and interpreting medical data, which is essential for supporting regulatory submissions and advancing product development.
- Quality Assurance: Implementing stringent quality control measures throughout the research process is a hallmark of Medtech CRO Services. This ensures the integrity and high standards of data, which are vital in medical research.
- Biostatistics: Providing robust statistical analysis and support, CROs help ensure that studies yield reliable and scientifically valid results.
- Study Project Management and Reporting: Effective coordination with clinical trial sites is critical for facilitating participant recruitment and maintaining compliance with study protocols. This encompasses extensive reporting on study status, inventory, and serious and non-serious adverse events, which are essential for ongoing project management and compliance. Clinical research organizations guarantee that all negative occurrences are recorded and communicated following compliance standards, which is vital for preserving the safety and effectiveness of the investigational products.
Medtech CRO Services are crucial to the efficient development of medical technologies, ensuring that they not only satisfy compliance requirements but also aid in the progress of healthcare. Notably, the regulatory and medical affairs segment within the CRO industry is projected to grow at a remarkable CAGR of 12.4%, reflecting the increasing trend of medical device companies outsourcing these essential functions. Additionally, the financial performance of contract research organizations, as evidenced by key points regarding Eurofins Scientific SE, underscores their market position and viability.
Furthermore, innovative applications, such as those highlighted in the case study on AI in medical imaging, exemplify the evolving landscape and capabilities of Medtech CROs.
Current Trends and Future Directions in Medtech CRO Services
The Medtech CRO landscape in Latin America is undergoing a significant transformation, shaped by key trends that highlight both challenges and solutions for Medtech companies:
- Increased Use of Technology: The integration of advanced digital tools and platforms for data collection and management has become essential, enhancing operational efficiency and data accuracy. This technological change enables more seamless cooperation between US Medtech companies and Latin American research locations, tackling the fragmentation of resources and communication obstacles apparent in the area.
- Patient-Centric Approaches: Emphasizing patient perspectives in the research process leads to customized clinical studies that foster higher engagement and relevance. By aligning with patient needs, these studies can achieve more meaningful outcomes, which is crucial in the context of Medtech innovations being tested in diverse environments like Colombia, as highlighted by PAVmed's First In-Human Study.
- Regulatory Changes: Continuous updates in regulatory frameworks necessitate that contract research organizations remain agile. Adherence to these changing regulations is essential for upholding the integrity of medical studies and ensuring patient safety, especially in managing the complexities of Latin American markets and aligning with local requirements.
- Globalization of Clinical Studies: As companies aim to broaden their market reach, CROs are conducting studies across various geographical locations. This globalization highlights the importance of understanding local regulations and cultural nuances, which can significantly enhance the effectiveness of research efforts in Latin America.
- Focus on Real-World Evidence: The shift towards utilizing real-world data enhances conventional study results and provides stakeholders with a detailed insight into product performance. This evidence is crucial for informed decision-making and emphasizes the influence of Medtech research studies on local economies, aiding in job creation, economic development, and enhanced healthcare services.
Moreover, the challenges encountered by US Medtech firms, such as professionalism, language barriers, and fragmentation of resources, must be tackled to promote better collaboration with Latin American research locations. Furthermore, the comprehensive clinical trial management services provided by contract research organizations, including feasibility studies, site selection, compliance reviews, and reporting, play a critical role in ensuring successful trial execution.
Moreover, the collaboration between Greenlight Guru and bioaccess™ exemplifies how strategic partnerships can accelerate Medtech innovations and clinical trials in Latin America, driving global health improvements through international collaboration.
These trends reflect a dynamic future for Medtech CRO Services, presenting ample opportunities for innovation and enhanced patient outcomes in clinical research. As only 13% of organizations currently prioritize digital transformation, embracing these evolving trends will be vital for Chief Revenue Officers to remain competitive and relevant in the ever-changing healthcare landscape.
Challenges and Considerations for Medtech CROs
Medtech Contract Research Organizations navigate a complex environment filled with challenges that can greatly influence their operational effectiveness. Among these challenges are:
-
Regulatory Compliance: The ever-evolving regulatory environment necessitates continuous education and training for staff to keep pace with changes.
With the MedTech industry witnessing advancements in AI and potential mergers, maintaining compliance has become increasingly intricate. The role of INVIMA, Colombia's National Food and Drug Surveillance Institute, is crucial here, as it provides oversight for medical devices and enforces regulations that align with PAHO/WHO standards, ensuring that clinical research organizations adhere to high-quality benchmarks.
-
Resource Management: Balancing multiple projects with limited resources often strains operational capabilities, particularly in light of the 14 therapeutic centers of excellence, including oncology, GI/NASH, pediatrics, neurology, and rare diseases, that demand focused attention.
This resource management challenge is critical as clinical research organizations strive to meet the diverse needs of these specialized areas while facilitating job creation and economic growth through local clinical studies.
-
Data Security: Protecting sensitive patient data is paramount, requiring robust cybersecurity measures. Recent reports have highlighted the urgency of addressing data security issues, as breaches can lead to significant operational and reputational damage, further complicating the compliance landscape.
-
Talent Acquisition and Retention: The competition for skilled personnel is fierce, making it challenging for CROs to attract and retain top talent. As the industry progresses, the need for specialized knowledge, especially in compliance matters as demonstrated by professionals like Katherine Ruiz, becomes more crucial.
-
Stakeholder Communication: Effective communication among diverse stakeholders—including sponsors and regulatory bodies—is essential for project success.
Transparent pathways for communication can reduce misunderstandings and promote smoother cooperation, which is essential in a field that depends on global collaboration and innovation to enhance worldwide health.
To tackle these issues, Medtech CRO Services provide extensive offerings such as feasibility studies to evaluate site viability, meticulous site selection to guarantee optimal study conditions, and project management to streamline operations. For instance, by conducting thorough feasibility studies, contract research organizations can identify the most suitable research sites and principal investigators (PIs), thereby enhancing the likelihood of successful trial outcomes.
Moreover, the economic impact of Medtech clinical studies on local economies is significant; these studies not only create jobs but also stimulate economic growth by fostering partnerships with local healthcare providers and institutions.
Addressing these challenges is crucial for the effectiveness and credibility of Medtech CRO Services in an increasingly competitive environment. Acknowledging the complexities highlighted by 88% of executives, who rated technological advancements as a top challenge, reflects the urgent need for strategic approaches to navigate these issues successfully. The challenges encountered by the MedTech sector, as detailed in the case study, further demonstrate the pressures that contract research organizations must contend with in this rapidly evolving landscape.
The Impact of Technology on Medtech CRO Services
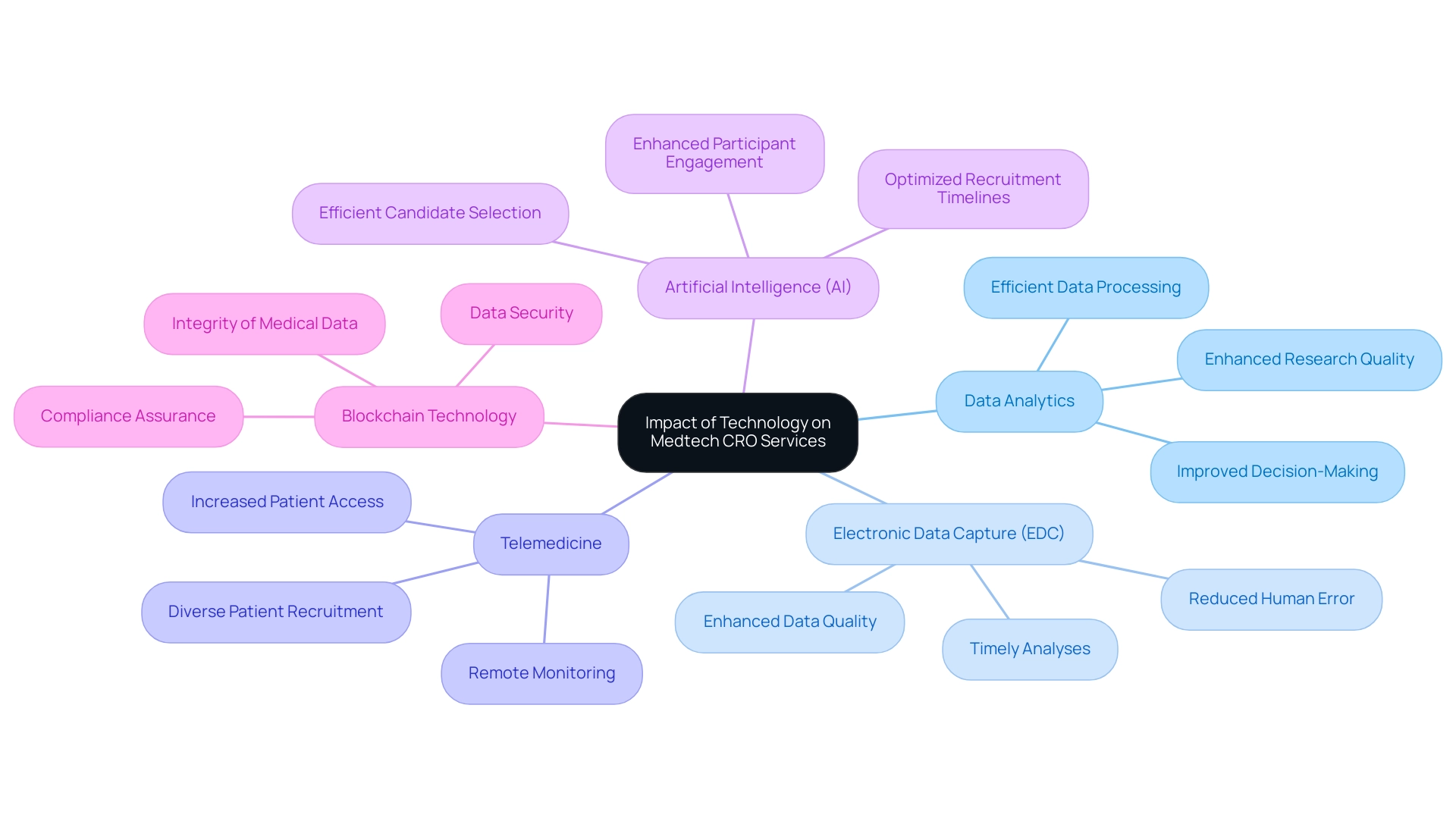
The transformative power of technology in Medtech CRO services is evident across multiple dimensions, exemplified by bioaccess®.
Data Analytics: The implementation of advanced data analytics tools allows Contract Research Organizations (CROs) like bioaccess® to efficiently process and analyze extensive volumes of clinical data. This capability not only speeds up the decision-making process but also improves the overall quality and reliability of research results, particularly in the context of cost-effective studies in Latin America.
Electronic Data Capture (EDC): EDC systems have emerged as essential components in streamlining data collection processes. By reducing the chance of human error and enhancing data quality, these systems enable more precise and prompt analyses, ultimately supporting the integrity of results. Bioaccess® utilizes these systems to ensure dependable and timely outcomes for startups utilizing Medtech CRO Services.
Telemedicine: The growth of telemedicine has transformed patient access to research studies, especially considering recent global health challenges. This innovation enables remote monitoring and participation, thereby broadening the recruitment base and ensuring diverse patient representation in studies, an area where bioaccess® excels in patient recruitment.
- Artificial Intelligence (AI): The incorporation of AI in medical studies has proven invaluable, especially in patient recruitment.
By leveraging AI algorithms, CROs can identify and select suitable candidates more efficiently, optimizing recruitment timelines and enhancing participant engagement. Bioaccess® implements these methods within its extensive trial management services.
Blockchain Technology: Blockchain offers promising solutions for protecting data security and integrity within research.
Its decentralized nature ensures that medical data remains tamper-proof, fostering trust among stakeholders and adherence to compliance standards, which is crucial in the approval processes bioaccess® manages. The convergence of these technologies is fundamentally reshaping the operational landscape of Medtech CRO Services, resulting in enhanced efficiency and improved outcomes in research. For instance, bioaccess®'s expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies showcases how innovative technologies can revolutionize the design and delivery of medical treatments.
Moreover, bioaccess® will quickly and reliably deliver regulatory approval, clinical research site activation, patient recruitment, and trial data, connecting innovative Medtech startups with top-ranked clinical research sites in Latin America, bringing certainty in:
- Moving to the next phase (an FDA IDE pivotal study)
- Raising funds
- A successful exit.
The partnership between security leader Dignari and EY, aimed at mission enablement in the public sector, underscores the critical need for technological interventions in healthcare.
This collaboration reflects the ongoing trend of integrating advanced technologies within the industry, as evidenced by the significant market capitalization growth of leading medical technology companies in 2020—Teladoc Health (139%), Novocure (105%), Align Technology (92%), Abiomed (90%), and West Pharmaceutical Services (88%). These developments align with insights from Abbaoui et al. (2024), which underscore the vital role of AI methods in precision healthcare, further emphasizing the necessity for CROs like bioaccess® to adapt and innovate in this evolving environment.
Conclusion
The role of Medtech Contract Research Organizations (CROs) in the medical technology landscape cannot be overstated. Their comprehensive services, ranging from regulatory affairs to clinical trial management, are crucial for navigating the complexities of the healthcare environment. With a focus on regulatory compliance and the integration of advanced technologies, CROs facilitate efficient clinical research processes that are essential for the successful development and commercialization of medical devices and therapies.
As highlighted throughout the article, the current trends such as increased utilization of technology, patient-centric approaches, and globalization of clinical trials are reshaping the operations of Medtech CROs. These trends not only enhance operational efficiency but also ensure that clinical trials are more aligned with patient needs, ultimately leading to improved healthcare outcomes. The challenges faced by these organizations, including regulatory compliance and data security, necessitate a strategic approach to maintain credibility and effectiveness in a competitive market.
Looking ahead, the future of Medtech CRO services appears promising as they continue to innovate and adapt to emerging challenges and opportunities. By embracing technological advancements and focusing on real-world evidence, CROs can further enhance their value proposition to stakeholders in the healthcare ecosystem. The collaboration between CROs and medical technology firms will be pivotal in driving innovation and improving patient care, ensuring that new medical technologies not only meet regulatory standards but also significantly impact global health.
Frequently Asked Questions
What are Medtech CRO Services?
Medtech CRO Services encompass a comprehensive range of outsourced research functions essential for the development and commercialization of medical technologies, including feasibility evaluations, investigator selection, compliance with regulations, project management for studies, and comprehensive reporting.
How do Medtech CRO Services assist medical device producers and pharmaceutical firms?
These services provide critical support through the review and feedback on study documents to ensure compliance with country requirements, obtaining import permits, and nationalizing investigational devices, thus facilitating the research process.
What impact have Medtech CRO Services had on clinical trial processes?
They have significantly reduced clinical trial subject recruitment time by over 50% and achieved a retention rate exceeding 95%, demonstrating their effectiveness in real-world scenarios.
Why is quality management important in Medtech?
Quality management is considered a catalyst for innovation in the MedTech sector, as it goes beyond regulatory requirements to drive advancements in medical technologies.
What role do Medtech CRO Services play in regulatory compliance?
They help clients navigate complex regulatory landscapes, ensuring compliance with local and international laws, and have established relationships with regulators to assist in the approval process.
What services are included in comprehensive clinical study management?
This includes planning, execution, and monitoring of clinical studies, feasibility assessments, site selection, and ensuring adherence to protocols and timelines, along with the preparation of necessary documentation for ethics committee approvals.
How do Medtech CRO Services handle import permits and nationalization of investigational devices?
They assist in securing necessary import permits and ensuring that investigational devices are nationalized, which facilitates smoother market transitions.
What is the significance of data management in Medtech CRO Services?
Data management involves collecting, analyzing, and interpreting medical data, which is essential for regulatory submissions and advancing product development.
How do Medtech CRO Services ensure quality assurance?
They implement stringent quality control measures throughout the research process to maintain the integrity and high standards of data, which are vital in medical research.
What is the projected growth rate for the regulatory and medical affairs segment within the CRO industry?
This segment is projected to grow at a compound annual growth rate (CAGR) of 12.4%, reflecting the increasing trend of medical device companies outsourcing essential functions.

