Introduction
The medical technology sector is undergoing a transformative phase, driven by the essential role of Contract Research Organizations (CROs) in facilitating clinical research and development. As Medtech companies strive to innovate and bring new devices to market, understanding the comprehensive services offered by CROs becomes paramount. From regulatory consulting and trial management to navigating the complexities of local and international guidelines, these organizations provide invaluable support that can significantly enhance the success of medical device initiatives.
This article delves into the intricacies of Medtech CRO services, particularly in the context of Panama, highlighting the opportunities, challenges, and future trends that shape this dynamic landscape. By exploring the strategic advantages of partnering with CROs, stakeholders can better appreciate how these collaborations foster innovation and improve patient outcomes in the ever-evolving healthcare environment.
Understanding Medtech CRO Services: Definition and Importance
Medtech CRO Services Panama include specialized assistance offered by Contract Research Organizations focused on the technology sector. These organizations, like bioaccess®, are essential to the development of healthcare devices, providing a complete range of services that encompass:
- Site feasibility
- Investigator selection
- Regulatory compliance evaluations
- Study setup
- Import permits
- Project management
- Detailed reporting
- Continuous post-market surveillance strategies
The importance of these services is emphasized by their capacity to streamline the research process, ensuring compliance with stringent regulatory standards while promoting the advancement of innovative healthcare solutions.
For example, bioaccess® excels in managing diverse study types, including:
- Early-feasibility studies
- First-in-human experiments
- Pilot studies
- Pivotal studies
- Post-market follow-up studies
These studies are all aimed at overcoming the unique challenges faced by device startups in Latin America, such as regulatory hurdles, competition from established players, recruitment issues, and financial constraints. Additionally, their implementation of ongoing post-market surveillance strategies is crucial in monitoring product safety and performance. This proactive approach not only ensures continuous improvement throughout the product lifecycle but also reinforces the product's quality, safety, and efficacy.
By combining assessment and strategy, Medtech CRO Services Panama, such as bioaccess®, improve the potential for product success in the marketplace. By collaborating with such organizations, companies can leverage their knowledge to maneuver through the intricacies of research studies efficiently, resulting in enhanced patient results and accelerated market entry for new medical devices. As highlighted by bioaccess®, 'Ready to enhance your research experience?'
Book a meeting with our team today and discover how we can drive your research forward,' showcasing the potential benefits of collaboration in this dynamic field.
Navigating the Medtech CRO Landscape in Panama: Opportunities and Challenges
The landscape of Medtech CRO Services Panama is characterized by a unique blend of opportunities and challenges. Strategically located in Central America, Panama features a strong healthcare infrastructure, establishing it as an appealing center for Medtech CRO Services Panama, which supports Medtech firms aiming to perform research in Latin America. The country features approximately 50% of the top hospitals in the region, according to annual rankings, which further bolsters its appeal.
As reported, the regulatory framework is undergoing significant enhancements aimed at streamlining processes and fostering collaboration among stakeholders, thus facilitating a more efficient environment for conducting research studies.
In this dynamic context, bioaccess® offers comprehensive management services for research, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
Notably, bioaccess® specializes in:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
These services are crucial for navigating the complexities of research in Panama. In comparison, Colombia has built a reputation as a knowledgeable and safe nation for conducting medical trials, enhancing the regional allure for Medtech initiatives.
Furthermore, there is a growing interest in Latin America for innovative medical technologies, particularly as European markets face regulatory challenges. However, navigating the local landscape is not without its challenges. Medtech companies often encounter obstacles such as:
- Adapting to evolving regulations
- Securing timely approvals
- Managing the varying levels of expertise among local research teams
As Julio Martinez-Clark, CEO and co-Founder of Bioaccess, aptly noted, 'Pay attention to the Pacific Alliance, pay attention to the OECD.'
This insight underlines the importance of being attuned to regional dynamics and opportunities. Grasping these complexities is vital for Medtech companies and CROs to effectively strategize their research initiatives in Panama, where Medtech CRO Services Panama can ensure successful project outcomes and leverage the potential of this burgeoning market. Moreover, partnerships like the one between Greenlight Guru and bioaccess™ seek to expedite Medtech advancements and trials in the area, illustrated by PAVmed's successful first-in-human trial in Colombia.
The impact of these clinical studies extends beyond research, creating jobs, driving economic growth, and improving healthcare in local communities, thereby enhancing international recognition.
Key Services Offered by Medtech CROs
Medtech CRO Services Panama offer a wide range of services tailored to meet the varied needs of device developers. Among the core offerings, regulatory consulting stands out as a crucial component, enabling clients to navigate the intricate web of device regulations and achieve compliance with both local and international standards. This is increasingly vital in a landscape where all stakeholders—including industry, researchers, patients, and payers—must collaborate to ensure the safety and innovation of healthcare technologies.
Notably, the Colombia National Food and Drug Surveillance Institute (INVIMA) plays a significant role in overseeing regulatory compliance, ensuring that devices meet rigorous safety and efficacy standards. Designated as a Level 4 health authority by the Pan American Health Organization/World Health Organization, INVIMA's roles encompass:
- Feasibility assessments
- Site selection
- Research setup
- Import permits
- Project management
- Monitoring
These are all vital components of Medtech CRO Services Panama.
In fact, recent research has demonstrated that regulatory consulting can significantly reduce the time to market for new medical devices, with some estimates suggesting enhancements of up to 30%. Furthermore, CROss excel in research design, creating protocols that improve the validity and reliability of study outcomes. Patient recruitment strategies are equally vital, as they guarantee the enrollment of appropriate participants, which is crucial for the success of any trial.
Effective data management and biostatistical analysis further enhance the credibility of research findings, making Medtech CRO Services Panama essential partners in the research process.
Furthermore, the impact of MedTech clinical research extends to local economies, fostering job creation, economic growth, and healthcare improvements, while also gaining international recognition for the regions involved. As noted by Nimesh Khadka, "Learn about the usage of process raman spectroscopy in the optimization of bioreactor monitoring and the improvement of cultivated meat production," which underscores the need for CROss to stay abreast of technological advancements that can optimize research outcomes. Additionally, a recent case study highlights that consulting fees can vary widely based on the consultant's experience, with rates ranging from $75 to $500 per hour, emphasizing the importance of weighing the costs of hiring a consultant versus training internal staff.
In summary, the multifaceted support provided by Medtech CRO Services Panama is essential for medical device developers aiming to successfully bring innovative products to market.
The Regulatory Environment for Medtech CROs in Panama
The regulatory framework overseeing Medtech CRO Services Panama is established by a mix of national and international guidelines, with the Panamanian Ministry of Health (MINSA) playing a crucial role in the approval and supervision of research activities. Adherence to Good Clinical Practice (GCP) guidelines is essential for CROs, requiring that all research activities obtain ethical approval from accredited local ethics committees. Furthermore, CROs must navigate the intricacies of local regulations, particularly those concerning informed consent procedures and data protection measures.
As the regulatory environment continues to evolve, it is imperative for Medtech CRO Services Panama and their clients to remain vigilant and informed about any regulatory changes. This proactive strategy assists in reducing risks linked to non-compliance and promotes the successful implementation of research studies. Recent position papers issued by MINSA emphasize the importance of adhering to established guidelines, particularly regarding the OECD Principles of GLP, which apply to non-clinical safety testing.
In this context, our Medtech CRO Services Panama encompass capabilities such as:
- Feasibility studies that assess the viability of research sites
- Site selection to ensure optimal locations for trials
- Compliance reviews to align with local regulations
- Trial setup that includes the preparation of necessary documentation and approvals
- Import permits for investigational devices
- Project management to oversee trial progress
- Reporting on study status, inventory, and adverse events
With expertise from professionals like Katherine Ruiz, a Regulatory Affairs specialist with substantial experience in health devices and in vitro diagnostics in Colombia, we offer valuable insights into navigating the complexities of regulatory requirements, thereby enhancing the success of research initiatives.
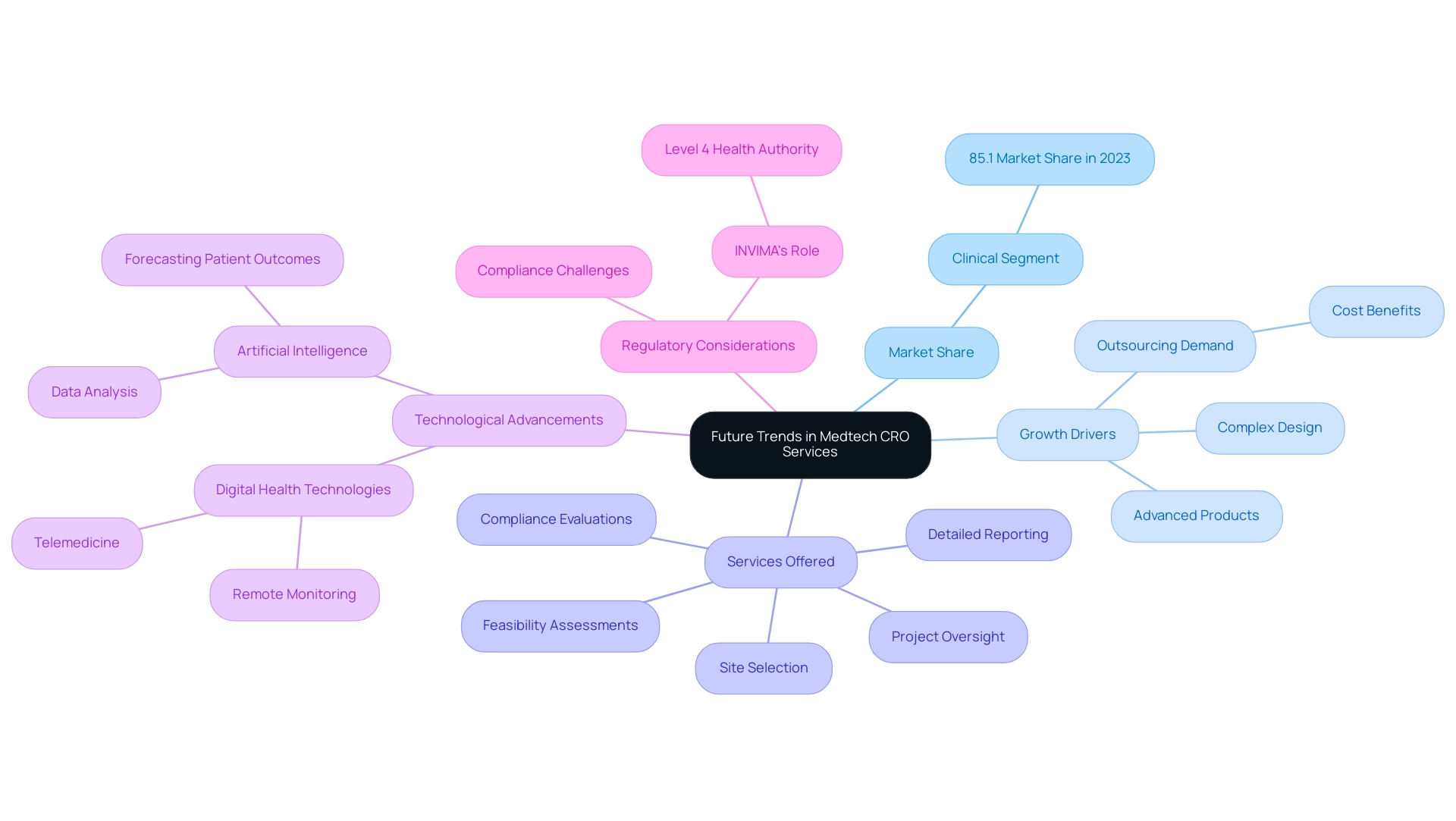
Future Trends in Medtech CRO Services
The future landscape of Medtech CRO Services Panama is poised for substantial evolution, largely influenced by technological advancements and dynamic regulatory frameworks. A substantial 85.1% of the device contract research organization market share was represented by the segment in 2023, highlighting the sector's significance. Key factors driving growth in the medical device CRO market include:
- Rising requirements for advanced products
- Increasing complexity in product design and engineering
- Growing outsourcing demand to emerging countries due to cost benefits
With more than 20 years of experience in the Medtech sector, bioaccess® distinguishes itself as a leader in this domain, providing extensive research management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Detailed reporting
Their expertise encompasses a wide range of studies such as Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, ensuring that investigations are navigated successfully through the regulatory landscape. bioaccess® utilizes specific methodologies that improve the efficiency and effectiveness of their research management processes.
The incorporation of digital health technologies, such as telemedicine and remote monitoring, is expected to greatly improve patient engagement and simplify data collection procedures during clinical studies. Furthermore, the growing acceptance of artificial intelligence and machine learning is anticipated to enhance more effective data analysis and informed decision-making; for example, AI algorithms can forecast patient outcomes and improve study designs. As regulatory bodies like INVIMA in Colombia evolve to accommodate these innovations, ensuring compliance becomes even more critical.
INVIMA functions as a Level 4 health authority, supervising the marketing and production of health products, which adds a layer of assurance for companies seeking to conduct studies in the region. Noteworthy developments, such as Laboratory Corporation of America Holdings' recent spin off to form an independent CRO and the collaboration between IQVIA and Alibaba Cloud, reflect the industry's trend toward enhancing clinical trial management. Additionally, bioaccess® offers a 15% free customization scope, equivalent to 5 analyst working days, for specific market information not currently included, highlighting the flexibility and responsiveness of the market.
By embracing these advancements and the economic impacts of Medtech studies—such as job creation and healthcare improvement—Medtech CRO Services Panama can secure a competitive advantage while continuing to support the development of pioneering medical technologies globally.
Conclusion
The exploration of Medtech CRO services reveals their critical role in supporting medical device development within Panama and beyond. By offering a wide array of essential services—including regulatory consulting, trial management, and post-market surveillance—CROs facilitate the complex journey of bringing innovative medical technologies to market. Their expertise not only helps navigate stringent regulatory landscapes but also enhances the potential for successful clinical outcomes, ultimately benefiting patients and healthcare systems alike.
As the Medtech landscape in Panama evolves, the unique opportunities presented by its strategic location and robust healthcare infrastructure become increasingly apparent. However, the challenges of adapting to regulatory changes and ensuring compliance remain significant. It is essential for stakeholders to remain informed and agile, leveraging the expertise of CROs to strategically navigate these complexities.
Looking ahead, the integration of advanced technologies and the continuous evolution of regulatory frameworks will further shape the future of Medtech CRO services. The anticipated growth driven by increasing demands for innovative medical devices underscores the importance of collaboration between Medtech companies and CROs. This partnership not only fosters innovation but also contributes to economic growth and improved healthcare outcomes in local communities.
In conclusion, the collaboration between Medtech companies and CROs is vital for overcoming the challenges of clinical research and enhancing the success of medical device initiatives. By embracing these partnerships, stakeholders can drive forward the development of groundbreaking technologies that ultimately improve patient care and health outcomes across the region.
Frequently Asked Questions
What are Medtech CRO Services Panama?
Medtech CRO Services Panama are specialized assistance provided by Contract Research Organizations focused on the technology sector, essential for the development of healthcare devices. They offer a comprehensive range of services including site feasibility, investigator selection, regulatory compliance evaluations, study setup, import permits, project management, detailed reporting, and continuous post-market surveillance strategies.
What types of studies do Medtech CROs like bioaccess® manage?
Bioaccess® excels in managing various study types, such as early-feasibility studies, first-in-human experiments, pilot studies, pivotal studies, and post-market follow-up studies.
Why are Medtech CRO Services important for device startups in Latin America?
These services help device startups navigate unique challenges such as regulatory hurdles, competition from established players, recruitment issues, and financial constraints, ultimately promoting the advancement of innovative healthcare solutions.
How does Panama's healthcare infrastructure support Medtech CRO Services?
Panama has a strong healthcare infrastructure, featuring approximately 50% of the top hospitals in the region, making it an appealing center for Medtech CRO Services aimed at supporting research in Latin America.
What role does regulatory consulting play in Medtech CRO Services?
Regulatory consulting is crucial for helping clients navigate complex device regulations and achieve compliance with local and international standards, which can significantly reduce the time to market for new medical devices.
What challenges do Medtech companies face in Latin America?
Medtech companies often encounter challenges such as adapting to evolving regulations, securing timely approvals, and managing varying levels of expertise among local research teams.
What are the key components of the regulatory framework governing Medtech CRO Services in Panama?
The regulatory framework is established by national and international guidelines, with the Panamanian Ministry of Health (MINSA) overseeing research activities. Compliance with Good Clinical Practice (GCP) guidelines and ethical approval from accredited local ethics committees are essential.
How do Medtech CRO Services impact local economies?
The impact of Medtech clinical research extends to local economies by fostering job creation, driving economic growth, and improving healthcare, while also enhancing international recognition for the regions involved.
What are the future trends expected in Medtech CRO Services Panama?
Future trends include the incorporation of digital health technologies, artificial intelligence, and machine learning, which are anticipated to enhance patient engagement, simplify data collection, and improve data analysis and decision-making in clinical studies.
How can companies leverage Medtech CRO Services for better research outcomes?
By collaborating with organizations like bioaccess®, companies can navigate the intricacies of research studies more efficiently, resulting in enhanced patient outcomes and accelerated market entry for new medical devices.

