Introduction
In the complex realm of clinical research, Serious Adverse Events (SAEs) represent a critical concern that can significantly impact patient safety and the integrity of trials. Defined as any undesirable experiences linked to medical products that result in severe consequences, such as hospitalization or even death, SAEs demand meticulous attention from researchers and regulatory bodies alike.
The implications of these events extend beyond individual patient experiences, influencing regulatory assessments and the overall landscape of medical product safety. As the industry evolves, understanding the nuances of SAE reporting, compliance requirements, and emerging technological advancements becomes essential for safeguarding patient welfare and ensuring the success of clinical trials.
This article delves into the multifaceted nature of SAEs, exploring their definition, the challenges associated with reporting, and the future directions of management tools designed to enhance research efficacy and regulatory compliance.
Understanding Serious Adverse Events (SAEs): A Fundamental Overview
Serious Adverse Events are defined as any undesirable experiences linked to the use of a medical product that result in significant harm, such as death, life-threatening situations, hospitalization, or disability. Numerous individuals encounter significant weakness and extended illness durations, frequently with partial recovery and persistent symptoms, emphasizing the serious consequences of serious adverse events in clinical research. These events directly affect not only patient safety but also the integrity of clinical trials.
Regulatory agencies such as the FDA and EMA require thorough documentation and analysis of Sales, ensuring the identification and mitigation of potential risks associated with medical products. Our service capabilities enhance this process through feasibility studies, compliance reviews, and trial setup, employing methodologies such as risk assessment and continuous monitoring to navigate the complexities OFSAA documentation. Additionally, the significance of documenting non-serious adverse events cannot be overlooked, as they contribute to a fuller understanding of product safety and efficacy.
Recent updates indicate that three methods currently limit analyses to Tier 2 adverse events, underscoring the evolving landscape of SAE documentation. Furthermore, efficient recognition and handling of serious adverse events are crucial, as they affect trial results and upcoming regulatory choices, ultimately influencing healthcare practices. As highlighted by Key MN, some diet and supplement trials have shown a staggering 67% increased risk of death (X (1, N = 2), p < 0.000197), emphasizing the critical nature of SAE documentation in safeguarding patient safety and maintaining regulatory compliance in clinical research.
Tools and Resources for SAE Definition: Enhancing Research and Compliance
A variety of SAE definition tools and resources are essential for defining and managing Serious Adverse Events. Standardized SAE definition tools, comprehensive databases for tracking adverse events, and advanced software solutions that facilitate the collection and analysis of SAE data play pivotal roles in this process. Moreover, our comprehensive clinical trial management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Review and feedback on study documents to comply with country requirements
This ensures that all aspects of trial setup, including import permits and project management, are meticulously addressed. The implementation of electronic data capture (EDC) systems stands out as a significant advancement, enabling real-time monitoring and efficient reporting of serious adverse events, allowing researchers to respond promptly to any critical incidents. Furthermore, regulatory guidance documents, such as the ICH E2A and the FDA's Risk Evaluation and Mitigation Strategies (REMS) requirements, provide crucial insights into the regulatory framework governing serious adverse events.
The incidence rate, determined by dividing the number of new cases by the total patient-years of exposure, functions as a quantitative measure of risk that is essential for comprehending the impact of serious adverse events over time. Moreover, training programs and workshops, like those provided by the NIH, are essential in informing clinical research teams about the significance of accurate SAE documentation and the efficient use of SAE definition tools, ultimately leading to improved compliance and patient safety. Such initiatives align with ongoing efforts to foster partnerships that improve diversity within the scientific workforce, thereby enriching the overall research environment.
The authors declare that this manuscript received funding from Galapagos NV, highlighting the importance of support in developing effective SAE definition tools. Katherine Ruiz, a specialist in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, provides invaluable insights to the management of serious adverse events, ensuring adherence to local regulations and enhancing overall trial integrity.
The Importance of Timely Reporting of SAEs
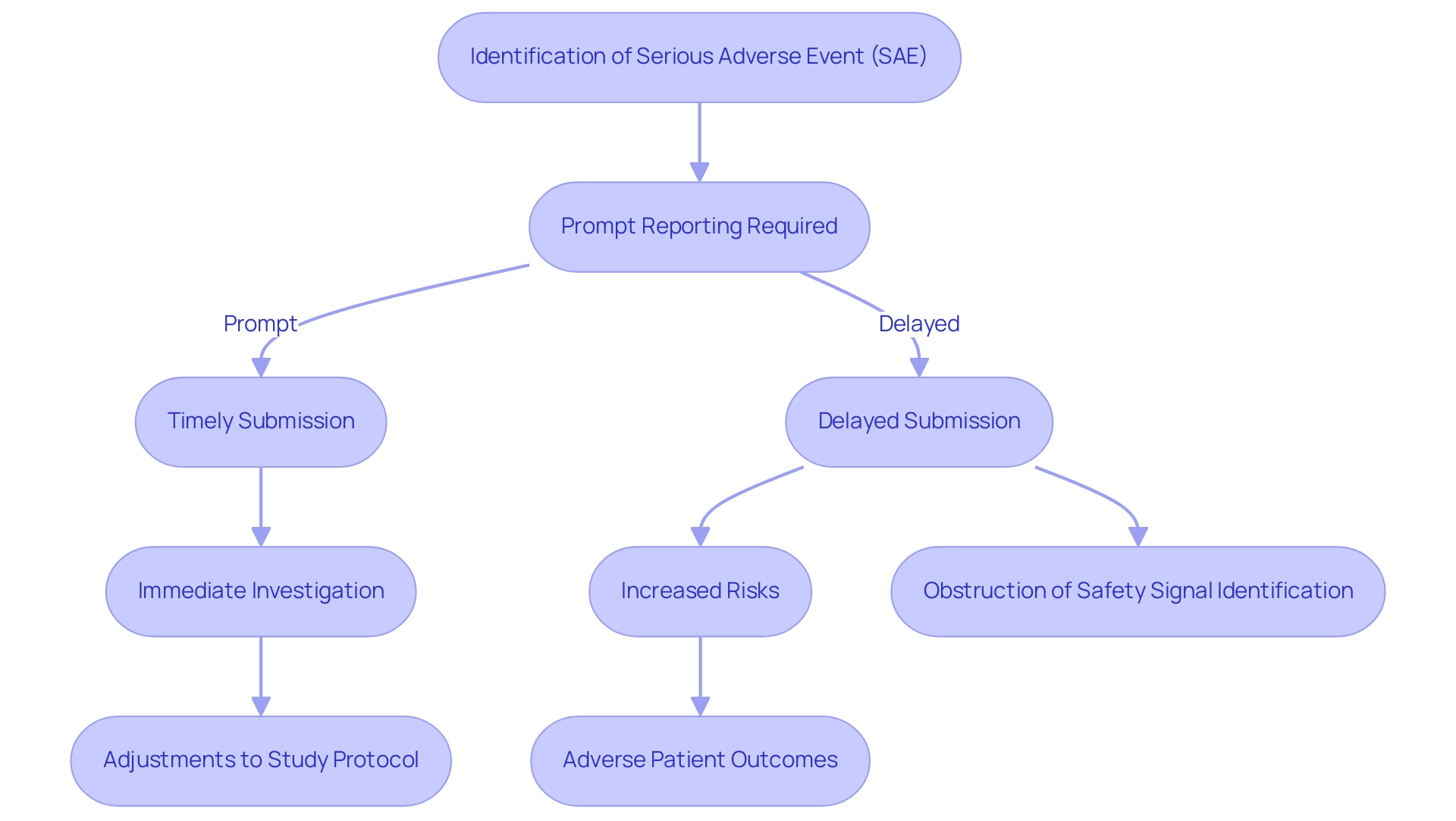
Prompt documentation of Serious Adverse Events (SAEs) is essential in the realm of clinical research, as delays can significantly impede regulatory assessments regarding the safety of medical products. With 70% of the population residing two hours or more from an academic medical institution, the difficulties of prompt communication are intensified, especially for individuals in isolated regions. Such delays not only heighten the risks encountered by individuals but can also obstruct the swift identification of potential safety signals.
For instance, if an unexpected SAE arises associated with a treatment, prompt notification is crucial for initiating immediate investigations and making necessary adjustments to study protocols, ultimately safeguarding participant welfare. The case study on Surgical Site Infections (SSIs) illustrates how delays in communication can adversely affect patient outcomes, emphasizing the real-world implications of this issue. Recent statistics indicate that delays in SAE definition tools documentation are a pressing concern, underscoring the need for efficient communication channels within research teams.
Our comprehensive clinical trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Project management
can significantly enhance the speed and accuracy of SAE submissions, ensuring that all relevant information is conveyed in a timely manner. Furthermore, our services encompass detailed examination and feedback on study documents, which are essential in guaranteeing compliance and enabling timely communication of both serious and non-serious adverse events. The Centers for Disease Control and Prevention indicates that effective communication and timely notification are essential in ensuring patient safety, further illustrating the critical nature of this issue within clinical research.
Challenges in SAE Definition and Reporting
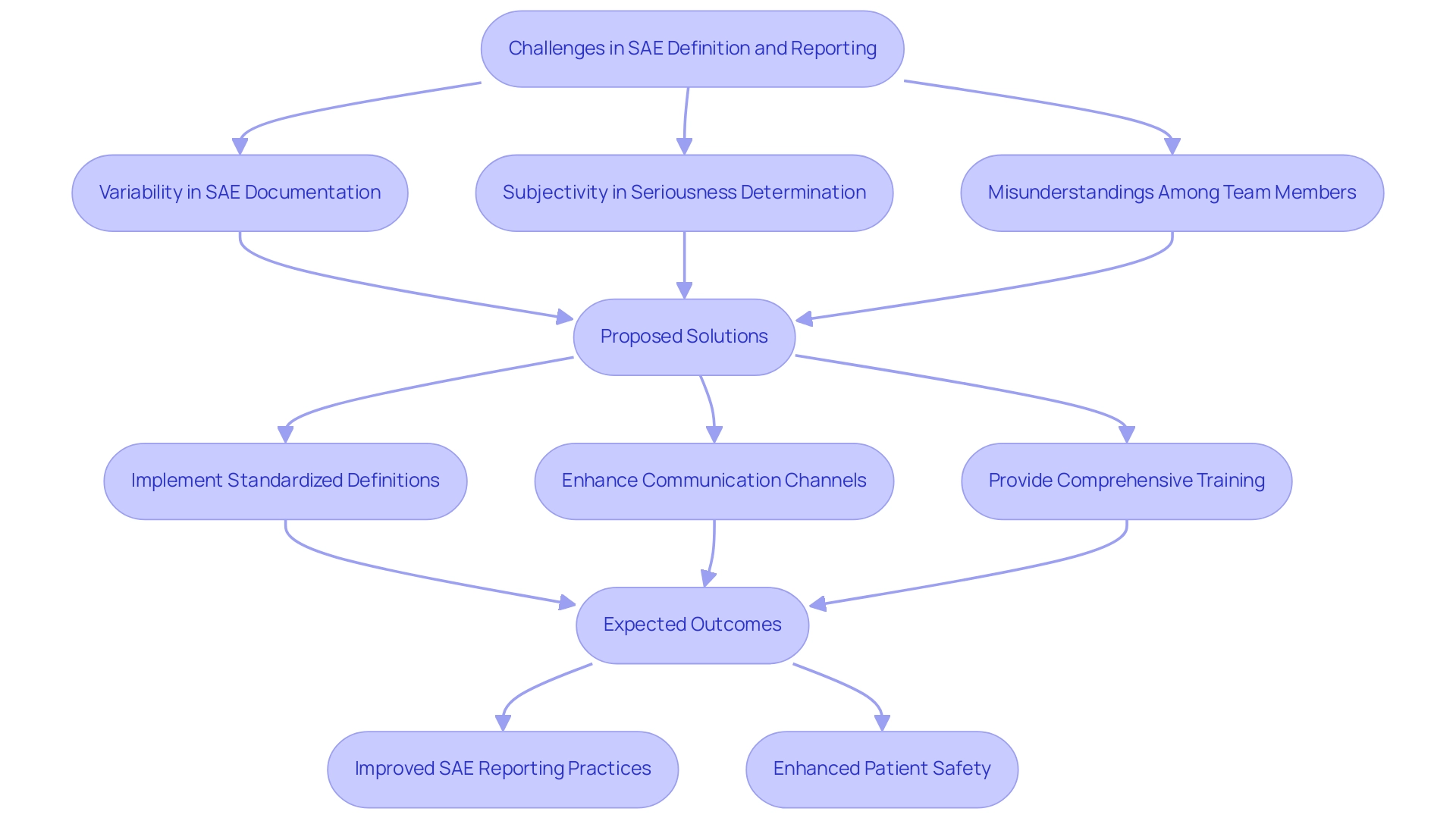
The use of sae definition tools for defining and documenting Serious Adverse Events (SAEs) presents several challenges that can impact the integrity of clinical trials. A primary concern is the variability in SAE documentation across different studies, leading to inconsistencies that complicate data collection and analysis. The subjective nature of certain adverse events further complicates the determination of what qualifies as serious.
Misunderstandings can happen not only among team members but also between researchers and sponsors, which may lead to delays or inaccuracies during documentation. In 2023, the ICH harmonized guideline on Good Clinical Practice (GCP) E6(R3) was published, highlighting the need for standardized definitions and sae definition tools to address these challenges. Furthermore, the system created within REDCap enables rapid and effective remote documentation and assessment of serious adverse events, minimizing mistakes and data replication.
Robin Rogiers notes, "A first vaccine against this coronavirus could still take some time to develop, but mRNA vaccine platforms could offer an early breakthrough," emphasizing the importance of SAE documentation in the context of vaccine development. Moreover, causality evaluations can be altered, prompting notifications for sponsors to examine Sales more swiftly, highlighting the significance of prompt reviews in the dynamic nature of SAE documentation. To enhance the quality of SAE documentation and ensure patient safety, it is essential to implement clear communication channels and provide comprehensive training for research teams.
A notable example of improvement in this area is the 'Highlight Changes' module, which visually indicates updated data points in Essay forms, making changes immediately noticeable. This innovation facilitates quicker assessments and enhances the overall review process. By fostering a culture of transparency and collaboration, organizations can significantly improve SAE reporting practices.
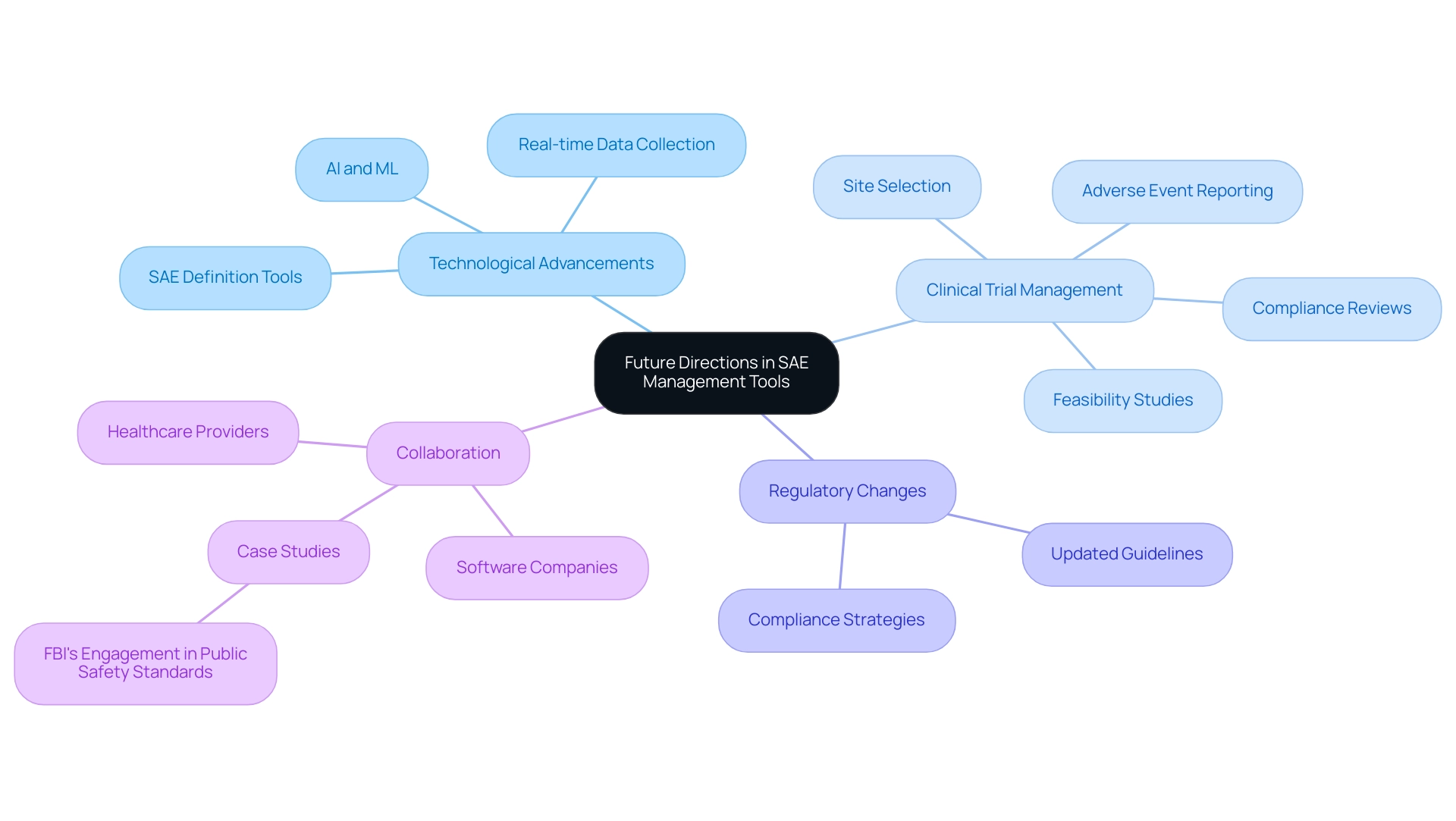
Future Directions in SAE Management Tools
The field of clinical research is undergoing significant transformation with the advent of advanced technologies, including SAE definition tools, in Serious Adverse Event (SAE) management. Artificial intelligence (AI) and machine learning (ML) are at the forefront of this evolution, enabling researchers to identify and analyze SAEs more effectively. These technologies facilitate the recognition of patterns and the prediction of potential adverse events, significantly enhancing proactive safety measures.
For instance, AI-driven models can process vast amounts of data to identify risk factors associated with specific treatments, thereby enhancing safety outcomes.
Moreover, our comprehensive clinical trial management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- The critical reporting of serious and non-serious adverse events
This ensures that trials are set up effectively and efficiently. The integration of mobile health technologies allows for real-time data collection through patient-reported outcomes, which are essential for SAE definition tools in monitoring.
This capability not only offers immediate insights into client experiences but also informs ongoing clinical assessments. As Sowmyalatha Jayaraman, Global Technical Specialist at General Motors, notes, "I am definitely looking forward to hearing from our software companies as to where they are at with their methods, development, and tools, development towards model-based systems engineering, and how they are applying AI and ML in this space more effectively." Collaboration between software companies and healthcare providers is essential for advancing these methodologies.
In light of these advancements, regulatory bodies are likely to reevaluate and update guidelines to accommodate the rapid pace of innovation in clinical research. Understanding these trends is vital for researchers and organizations aiming to remain compliant while ensuring the highest standards of patient safety. Notably, the recent $1.5 billion grant program administered by the National Telecommunications and Information Administration, funded by the CHIPS and Science Act of 2022, highlights the significant investment in technological advancements that support the development of SAE definition tools.
Additionally, case studies, such as the FBI's engagement in public safety standards, underscore the importance of collaboration in developing effective frameworks that utilize SAE definition tools to address emerging challenges in SAE management. With the landscape continually evolving, staying abreast of technological advancements and regulatory changes will be crucial for the successful navigation of SAE management, particularly through the implementation of SAE definition tools to enhance local economies via job creation and healthcare improvement in the coming years.
Conclusion
Understanding Serious Adverse Events (SAEs) is essential for maintaining patient safety and the integrity of clinical trials. These events, which can lead to severe consequences such as hospitalization or even death, require rigorous reporting and management to mitigate risks associated with medical products. The article has highlighted the critical role of timely SAE reporting, the challenges inherent in defining and documenting these events, and the importance of utilizing advanced tools and resources to enhance compliance and research efficacy.
The discussion on the future directions of SAE management tools underscores the transformative impact of technology, particularly artificial intelligence and machine learning, on the identification and analysis of SAEs. By leveraging these innovations, researchers can better predict adverse events and implement proactive safety measures, ultimately improving patient outcomes. Furthermore, the integration of mobile health technologies facilitates real-time monitoring and data collection, enhancing the overall effectiveness of clinical trials.
In conclusion, as the landscape of clinical research continues to evolve, it is imperative for stakeholders to remain vigilant in their approach to SAE management. By prioritizing accurate reporting, fostering clear communication, and embracing technological advancements, the industry can not only safeguard patient welfare but also uphold the highest standards of regulatory compliance. Continuous education and collaboration will be key in navigating the complexities of SAEs, ensuring that clinical trials contribute positively to the advancement of medical science and patient care.
Frequently Asked Questions
What are Serious Adverse Events (SAEs)?
Serious Adverse Events are undesirable experiences linked to the use of a medical product that result in significant harm, such as death, life-threatening situations, hospitalization, or disability.
How do Serious Adverse Events impact clinical research?
SAEs directly affect patient safety and the integrity of clinical trials, as they can lead to significant weakness, extended illness durations, and persistent symptoms in individuals.
What are the regulatory requirements for documenting SAEs?
Regulatory agencies like the FDA and EMA require thorough documentation and analysis of SAEs to identify and mitigate potential risks associated with medical products.
What services are offered to enhance SAE documentation and management?
Services include feasibility studies, compliance reviews, trial setup, risk assessment, and continuous monitoring to navigate the complexities of SAE documentation.
Why is it important to document non-serious adverse events?
Documenting non-serious adverse events contributes to a fuller understanding of product safety and efficacy.
What recent updates have been made regarding SAE documentation?
Recent updates indicate that three methods currently limit analyses to Tier 2 adverse events, reflecting the evolving landscape of SAE documentation.
What is the significance of recognizing and handling SAEs efficiently?
Efficient recognition and handling of SAEs are crucial as they affect trial results, regulatory choices, and ultimately influence healthcare practices.
What tools and resources are essential for managing SAEs?
Essential tools include standardized SAE definition tools, comprehensive databases for tracking adverse events, and advanced software solutions for data collection and analysis.
What advancements have been made in SAE management?
The implementation of electronic data capture (EDC) systems allows for real-time monitoring and efficient reporting of SAEs, enabling prompt responses to critical incidents.
How is the incidence rate of SAEs calculated?
The incidence rate is determined by dividing the number of new cases by the total patient-years of exposure, serving as a quantitative measure of risk over time.
What role do training programs play in SAE documentation?
Training programs and workshops inform clinical research teams about the significance of accurate SAE documentation and the use of SAE definition tools, improving compliance and patient safety.
Who provides insights into the management of serious adverse events?
Katherine Ruiz, a specialist in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, provides invaluable insights to ensure adherence to local regulations and enhance trial integrity.




