Introduction
The successful execution of clinical trials hinges on the precise definition and management of study endpoints, which serve as crucial indicators of treatment efficacy. These endpoints not only guide the design and statistical analysis of trials but also play a pivotal role in regulatory approvals and the overall validity of clinical findings.
As the landscape of clinical research evolves, understanding the nuances of primary, secondary, and exploratory endpoints becomes increasingly important. Furthermore, the integration of innovative technologies and patient-centric approaches is reshaping how data is collected and analyzed, ensuring that the voices of participants are heard and valued.
This article delves into the multifaceted nature of study endpoints, exploring their classifications, the significance of endpoint adjudication, and the regulatory considerations that must be navigated to align with health authority expectations. Through a comprehensive examination of these elements, the article aims to illuminate the foundational role that well-defined endpoints play in advancing clinical research and improving patient outcomes.
Defining Study Endpoints: The Foundation of Clinical Trials
Research markers function as conclusive indicators or results that evaluate the efficacy of a treatment, which raises the question of what are study endpoints in medical studies. With extensive research management services, including feasibility studies, site selection, and compliance reviews, these objectives become crucial in assessing the validity of a research hypothesis and offering a foundation for conclusions derived from the study. The trial setup procedure, encompassing ethics committee approvals and import permits, is crucial for creating a framework within which these targets can be accurately measured.
Outcomes can be categorized into two primary types:
- Clinical outcomes, such as survival rates and disease progression
- Surrogate outcomes, which might involve changes in biomarkers
A comprehensive grasp of these points is crucial, as they not only influence the structure of the research but also guide the statistical analysis methods used. Furthermore, the identification of limits can significantly influence regulatory decisions regarding the approval of treatments.
Recent insights suggest that categorizing participants based on pandemic-related factors can enhance standard analyses, particularly in the context of premature treatment discontinuation, which is only considered pandemic-related when directly linked to such factors. This organized method guarantees that outcomes thoroughly represent what are study endpoints and reflect the treatment's possible effectiveness. Additionally, the role of rigorous project management and reporting practices, including the monitoring of serious and non-serious adverse events, in defining and overseeing what are study endpoints cannot be overstated.
According to the research referenced by PMID 34191971, J.D. was responsible for background drafting and manuscript polishing, highlighting the importance of thorough preparation in medical research. Furthermore, a case examination titled 'Feature Engineering for Complexity Models' illustrates how classifying features into baseline and design components can improve the reliability of analysis and pinpoint factors affecting duration.
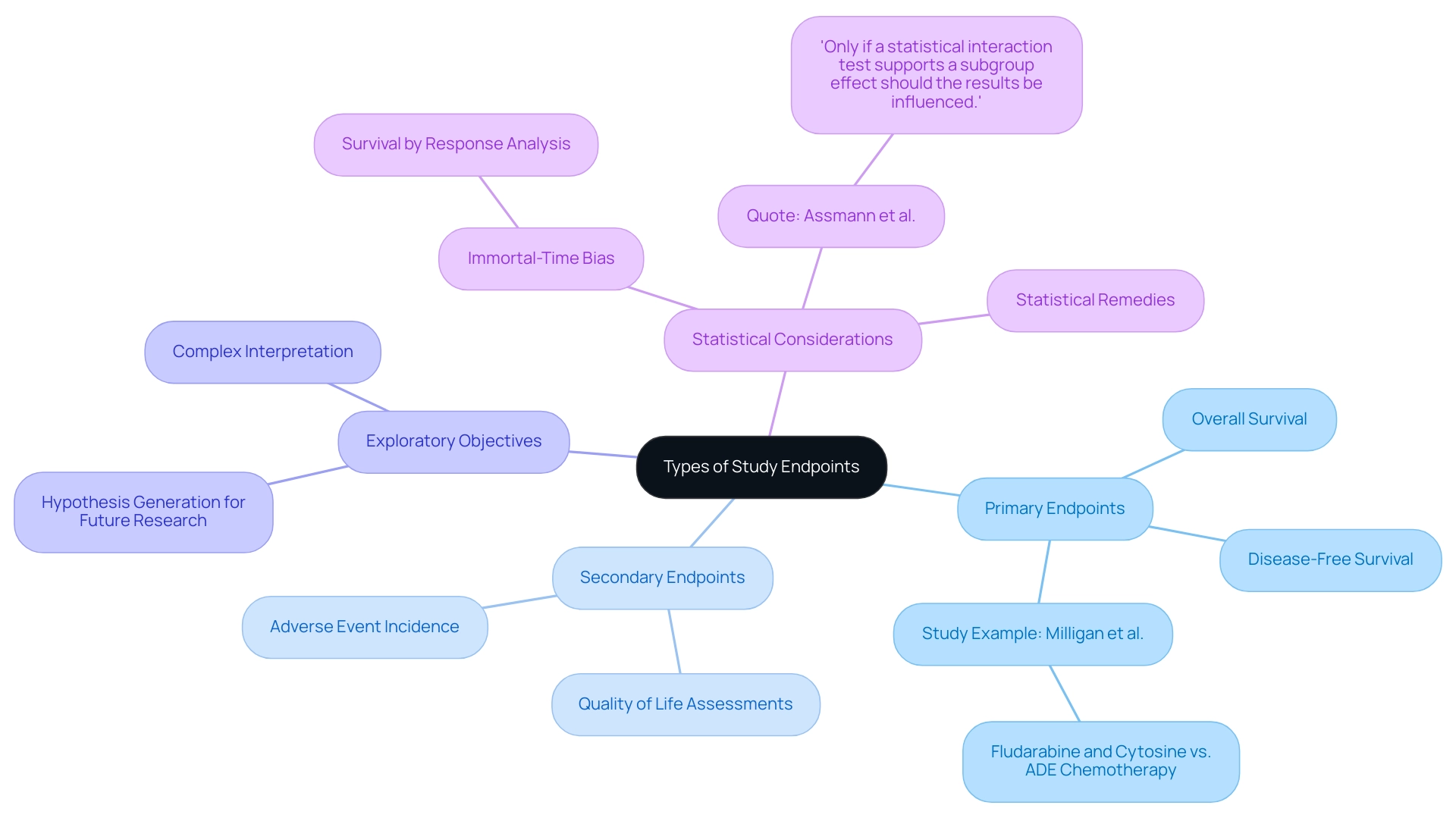
Types of Study Endpoints: Primary vs. Secondary and Beyond
In clinical evaluations, understanding what are study endpoints is essential as research goals are fundamentally categorized into primary and secondary classifications. Primary objectives signify what are study endpoints, which are the principal outcomes that a trial is specifically designed to evaluate, typically focusing on metrics such as overall survival or disease-free survival rates. For instance, a study by Milligan et al. discovered that fludarabine and cytosine were less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, emphasizing the significance of clearly defined primary goals.
In contrast, secondary measures provide supplementary insights regarding the treatment’s efficacy and safety, which raises the question of what are study endpoints, encompassing aspects such as quality of life assessments and the incidence of specific adverse events. Moreover, exploratory objectives may be incorporated into the research framework to create hypotheses for future investigations, although their interpretation is inherently more intricate and often necessitates careful consideration of potential biases.
A prevalent case examination in medical literature highlights the issues surrounding survival by response analysis, where comparisons between responders and non-responders can be skewed by immortal-time bias—only those who survive long enough to respond are included in the analysis. Statistical remedies exist to adjust for such biases, but the intricacies involved necessitate a nuanced understanding of the results. As noted by Assmann and colleagues, 'Only if a statistical interaction test supports a subgroup effect should the results be influenced.'
This differentiation among points is essential for understanding research results and their following consequences for medical practice, especially as the field of medical investigation keeps changing.
The Role of Endpoint Adjudication in Ensuring Trial Integrity
Endpoint adjudication is a crucial process within clinical studies, involving an independent review of what are study endpoints to ensure that outcomes are accurately reported and assessed. This systematic method is essential for minimizing bias and confirming that results genuinely reflect treatment effects. Typically, adjudication panels consist of experts who evaluate cases according to predefined criteria, thereby enhancing the reliability of the collected data.
The importance of this practice is highlighted by recent discussions in the field, with experts stating,
We need more precision about cardiovascular events for patients in oncology studies, and dedicated adjudication of cardiovascular events can provide that precision.
This increasing acknowledgment of case resolution as a standard procedure is especially apparent in intricate analyses, where personal interpretations can differ significantly. Significantly, the 2017 Cardiovascular and Stroke Outcome Definitions set guidelines intended to standardize outcome reporting and enhance the consistency of research results.
This case study demonstrates the development and standardization of reporting at the conclusion of research studies. Furthermore, a risk-based approach to adjudication is emerging, allowing for a balance between cost and accuracy by evaluating a smaller proportion of events initially and conducting further adjudication when inconsistencies arise. Comparisons of central adjudication of outcomes versus onsite assessment have shown that central adjudication can provide more accurate treatment effect estimates, thereby reinforcing the importance of independent review processes in ensuring study integrity.
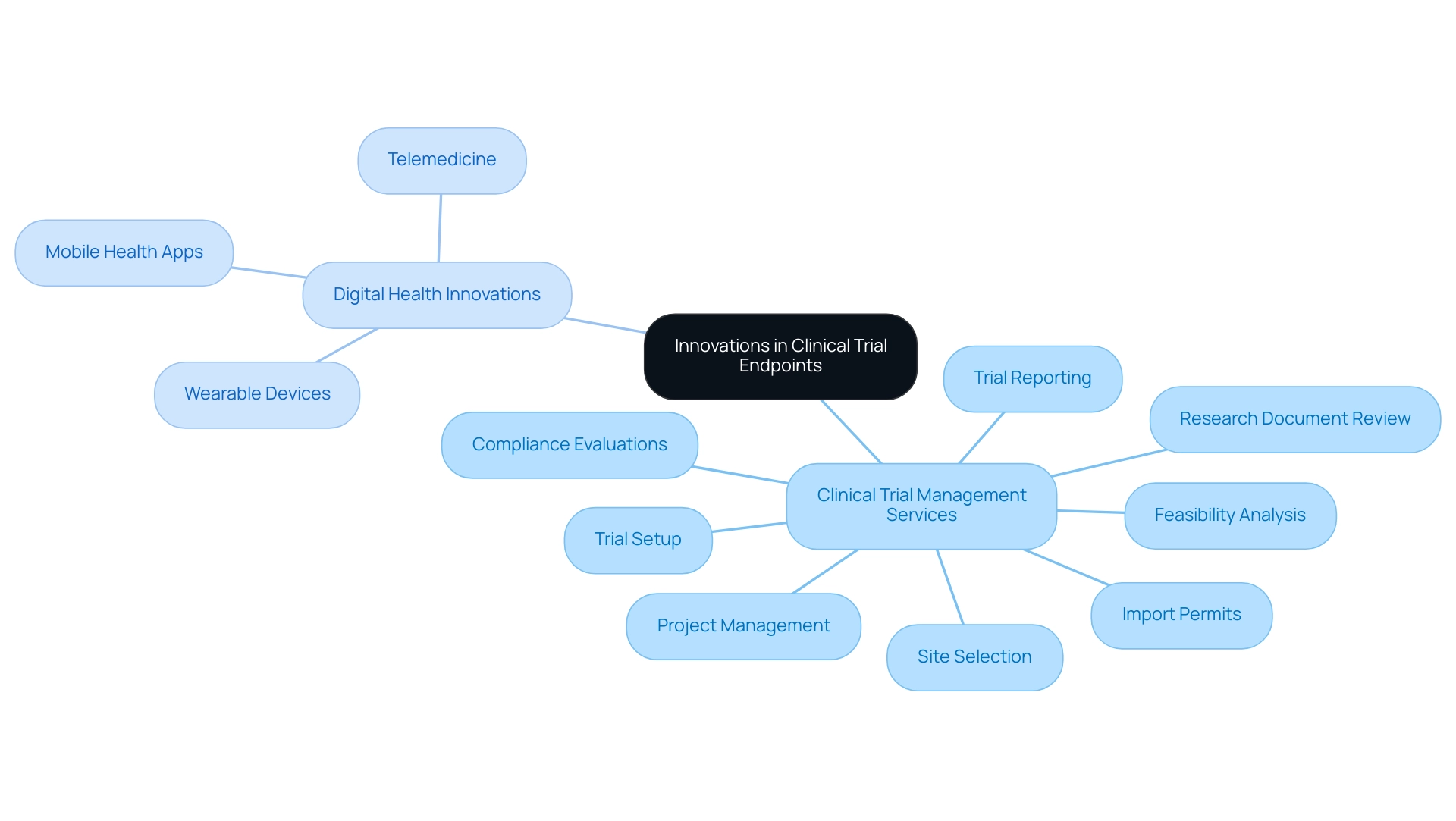
Innovations in Clinical Trial Endpoints: Embracing Digital Health and Patient-Centric Approaches
Recent advancements in digital health technologies have significantly transformed the framework of research objectives. Our extensive clinical trial management services encompass:
- Feasibility analysis
- Site selection
- Compliance evaluations
- Trial setup
- Review and feedback on research documents
- Import permits
- Project management
- Reporting on trial status and adverse events
These services are designed to address the multifaceted challenges faced by Medtech companies in Latin America.
The integration of wearable devices, mobile health applications, and telemedicine facilitates real-time data collection, enhancing the ability to capture patient-reported outcomes. This shift towards patient-centric approaches prioritizes the experiences and preferences of participants, ultimately leading to a clearer understanding of what are study endpoints that are more relevant to those affected by specific conditions. As noted by Dr. Pablo Cure, 'To reach the next level in the translation and implementation of DHTs, it is imperative to design and conduct rigorous, equitable, needs-based research and the corresponding validation efforts to generate the necessary evidence to support their use in biomedical research and health care for the benefit of all.'
These innovations not only enhance the quality of data but also promote greater patient involvement in research, which is vital for economic growth and healthcare advancement in the region. A review of articles published between January 2012 and July 2022 reveals a notable gap in funded projects focusing on health outcomes, emphasizing the need for further exploration in this area. Furthermore, a case examination monitoring trends in digital health publications and research activities from 2015 to 2023 shows a notable rise in both fields, especially during the pandemic, demonstrating a wider acceptance and incorporation of digital health technologies in biomedical research.
Regulatory Considerations: Aligning Study Endpoints with Health Authority Expectations
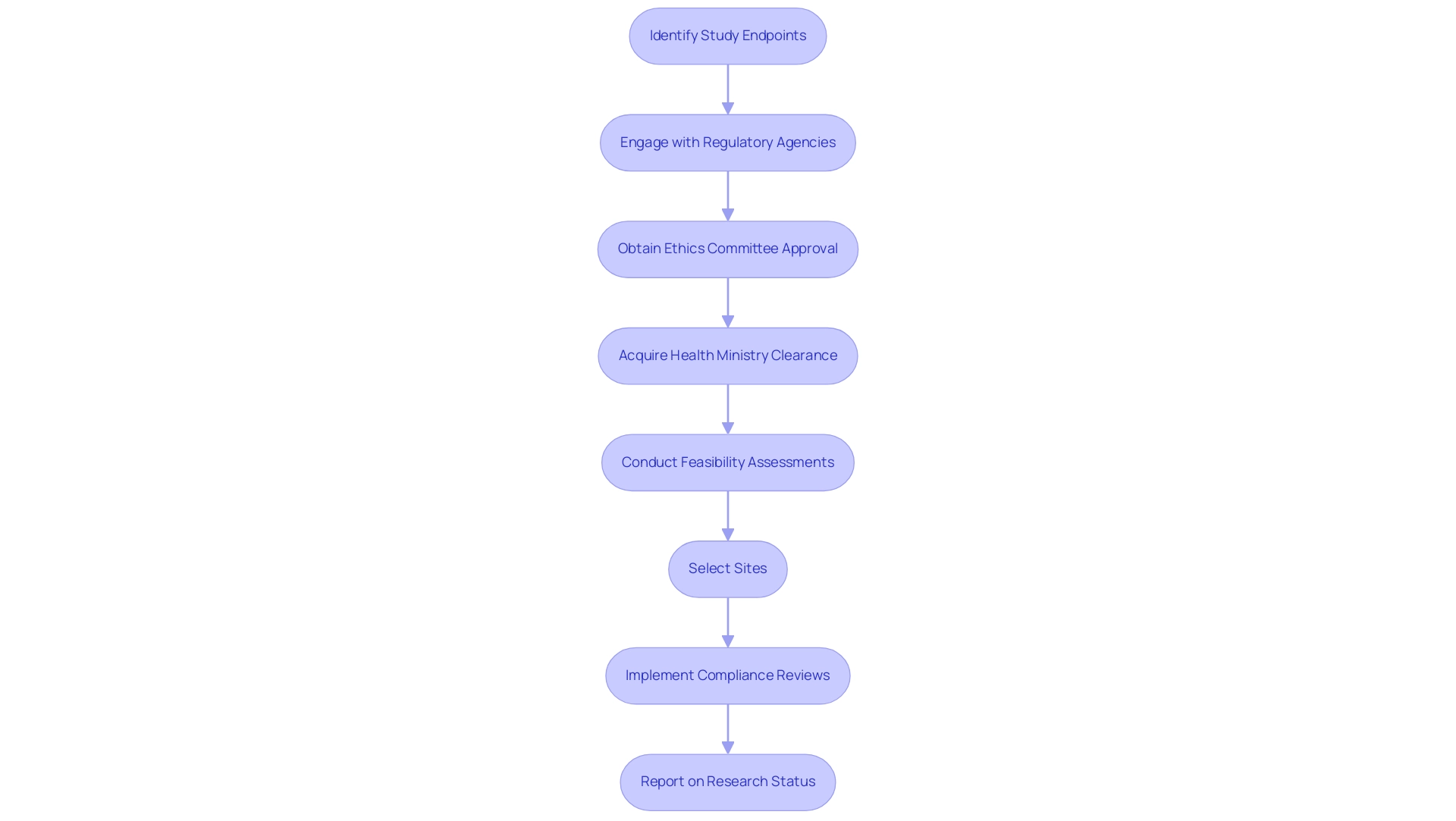
Regulatory factors are essential in understanding what are study endpoints and in the choice and specification of study objectives in clinical studies. Health authorities, including the FDA and EMA, provide specific guidelines that delineate criteria for acceptable outcomes. Aligning these points with regulatory expectations is crucial for successful study approval, as they must demonstrate tangible benefits to patients.
This alignment necessitates proactive engagement with regulatory agencies during the design phase to confirm what are study endpoints that meet the required evaluative standards. A failure to follow these guidelines can result in considerable delays or complete rejection of application submissions.
Our extensive clinical management services encompass feasibility assessments, site selection, and compliance reviews, guaranteeing that all elements of setup conform to regulatory standards, including acquiring necessary import permits. This process involves detailed procedures such as obtaining ethics committee approval and health ministry clearance, which are essential for the initiation of trials. Additionally, our services encompass thorough project management and oversight to ensure that research remains on track and compliant throughout their duration.
Reporting on research status, inventory, and serious and non-serious adverse events is also a critical component of our offerings.
The recent draft ICH E9(R1) addendum on estimands and sensitivity analysis, documented in a 324.57 KB PDF, highlights the evolving nature of these regulatory frameworks. Additionally, the case study titled 'Overview of Comments on Draft ICH E9(R1) Addendum' summarizes stakeholder feedback, offering valuable insights that can guide future revisions and enhancements to the guidelines on statistical principles for research studies.
Blake Wilson rightly points out,
If you have any questions about what are study endpoints in a research study or how to categorize them, or about the ability to draw conclusions from multiple endpoints in a research study, please do not hesitate to reach out to any of the authors of this alert or the Hogan Lovells attorney with whom you typically collaborate.
This underscores the importance of clear communication and understanding of regulatory expectations, which are essential for steering clinical trials toward successful outcomes. With the expertise of professionals like Ana Criado, Director of Regulatory Affairs, and Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, our team is equipped to navigate the complexities of regulatory compliance effectively.
Conclusion
The examination of study endpoints reveals their indispensable role in the successful execution of clinical trials. From defining primary and secondary endpoints to understanding the importance of endpoint adjudication, the article highlights how these markers are critical in assessing treatment efficacy and regulatory compliance. The integration of innovative technologies and patient-centric approaches further underscores the evolution of clinical trials, enabling more accurate data collection and fostering participant engagement.
Moreover, aligning study endpoints with regulatory expectations is paramount for facilitating successful trial approvals. Engaging with health authorities early in the trial design process ensures that endpoints meet the necessary evaluative standards, ultimately benefiting patient outcomes. The insights provided throughout the article emphasize that well-defined and meticulously managed study endpoints are not merely technical requirements; they are foundational elements that advance clinical research and improve healthcare delivery.
As the landscape of clinical research continues to evolve, the significance of robust endpoint definitions and management will only grow. Embracing these practices will lead to more reliable trial outcomes, drive innovation in treatment development, and enhance the overall integrity of clinical research. The ongoing dialogue among stakeholders, regulatory bodies, and researchers will be crucial in shaping the future of clinical trials, ensuring that they remain relevant and effective in addressing the complexities of modern healthcare challenges.
Frequently Asked Questions
What are study endpoints in medical studies?
Study endpoints are conclusive indicators or results that evaluate the efficacy of a treatment, serving as crucial objectives in assessing the validity of a research hypothesis.
What types of outcomes are categorized in medical research?
Outcomes are categorized into two primary types: clinical outcomes (such as survival rates and disease progression) and surrogate outcomes (which may involve changes in biomarkers).
Why is understanding study endpoints important in clinical evaluations?
Understanding study endpoints is essential because they are fundamentally categorized into primary and secondary objectives, which help define the principal outcomes a trial is designed to evaluate, influencing the structure of research and statistical analysis methods.
What are primary and secondary objectives in research?
Primary objectives signify the main outcomes a trial is designed to evaluate, typically focusing on metrics like overall survival. Secondary objectives provide supplementary insights regarding treatment efficacy and safety, such as quality of life assessments and the incidence of specific adverse events.
How can exploratory objectives be utilized in research?
Exploratory objectives may be incorporated to create hypotheses for future investigations, although their interpretation is more complex and requires careful consideration of potential biases.
What is the significance of rigorous project management in defining study endpoints?
Rigorous project management and reporting practices are vital for defining and overseeing study endpoints, including monitoring serious and non-serious adverse events.
What is the impact of pandemic-related factors on study analyses?
Categorizing participants based on pandemic-related factors can enhance standard analyses, particularly concerning premature treatment discontinuation, ensuring outcomes accurately represent study endpoints and reflect treatment effectiveness.
What is immortal-time bias in survival analysis?
Immortal-time bias occurs when comparisons between responders and non-responders are skewed because only those who survive long enough to respond are included in the analysis, necessitating statistical adjustments for accurate interpretation.
How do statistical interaction tests influence research results?
According to Assmann and colleagues, only if a statistical interaction test supports a subgroup effect should the results be influenced, highlighting the importance of nuanced understanding in research outcomes.




