Overview
The benefits of Latin America CROs (Contract Research Organizations) include significant cost savings, expedited regulatory processes, and access to diverse patient populations, which enhance the efficiency of clinical research. The article supports this by detailing how countries like Colombia offer over 30% savings compared to North America, swift regulatory reviews, and a robust healthcare system that facilitates patient recruitment, thereby positioning Latin America as an attractive destination for global research trials.
Introduction
The role of Contract Research Organizations (CROs) in Latin America has become increasingly vital in the realm of clinical research, serving as essential facilitators for pharmaceutical and biotechnology companies. These organizations provide a broad array of services that streamline the complexities of clinical trials, from study design to regulatory compliance.
As the landscape of global clinical research shifts, Latin America emerges as a compelling hub, particularly Colombia, which offers significant benefits such as:
- Reduced costs
- Expedited regulatory processes
- A robust healthcare system
With a diverse population and supportive government initiatives, the region not only enhances patient recruitment but also fosters an environment ripe for innovation and collaboration.
As the demand for clinical trials continues to grow, understanding the unique advantages and challenges faced by CROs in this dynamic landscape is crucial for stakeholders aiming to navigate the intricacies of clinical research effectively.
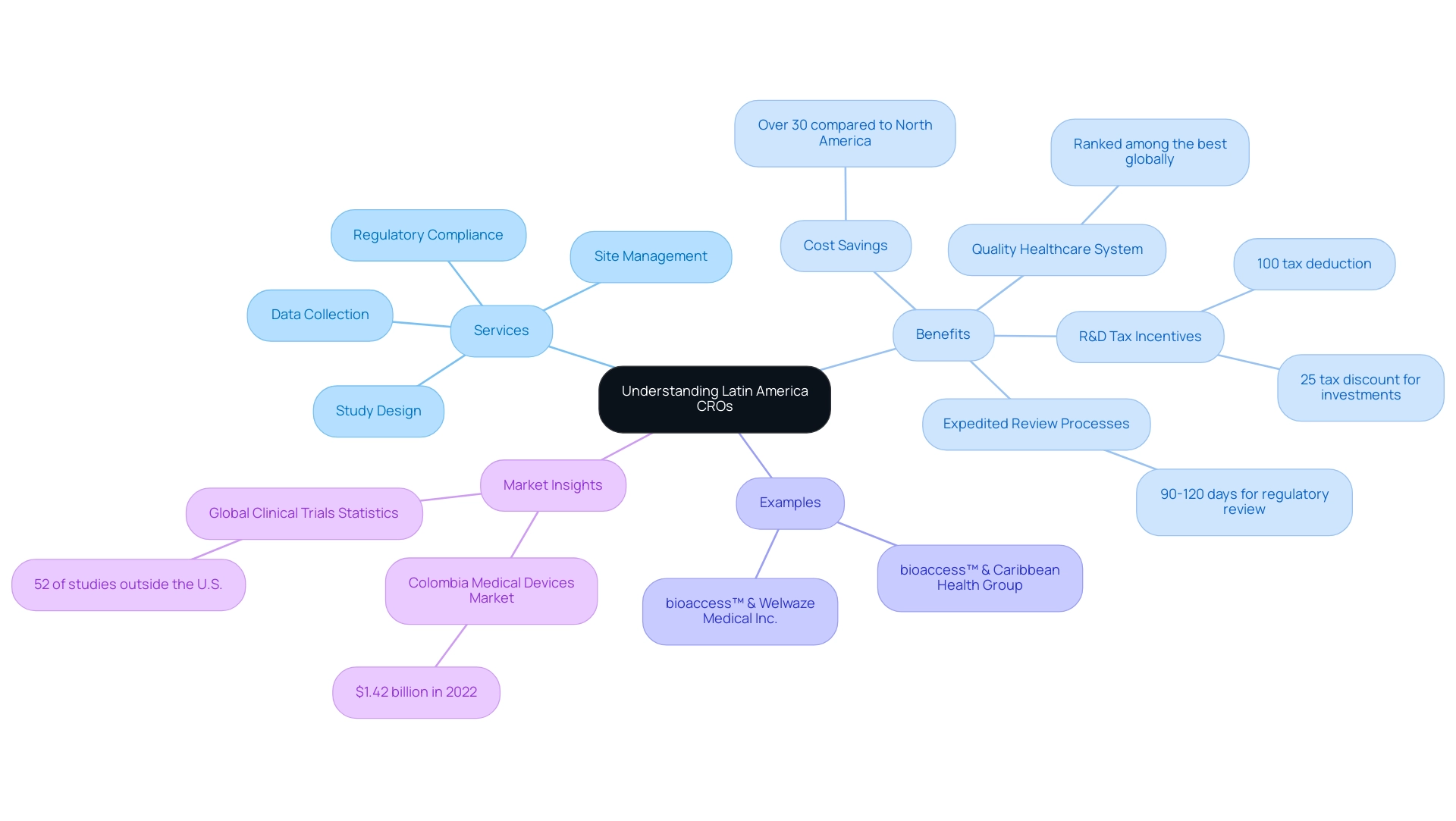
Understanding Latin America CROs: Definition and Role in Clinical Research
Contract Research Organizations (CROs) provide significant Latin America CRO benefits as vital participants in the oversight and implementation of research studies for pharmaceutical and biotechnology companies. These organizations offer a comprehensive suite of services, including:
- Study design
- Site management
- Data collection
- Regulatory compliance
By leveraging local knowledge and resources, the Latin America CRO benefits can improve the efficiency of research while adhering to international standards.
Colombia stands out as a prime location for first-in-human studies, showcasing the Latin America CRO benefits, which include:
- Cost savings of over 30% compared to North America and Western Europe
- Expedited regulatory review processes that typically take only 90-120 days
- A high-quality healthcare system ranked among the best globally
The rigorous ICH/GCP certification process that hospitals must undergo before conducting research ensures high-quality standards. The large and diverse population of over 50 million, with about 95% covered by universal healthcare, further facilitates effective patient recruitment.
Moreover, the R&D tax incentives offered in Colombia, featuring a 100% tax deduction and a 25% tax discount for investments in science, technology, and innovation, are key examples of Latin America CRO benefits that provide significant financial advantages for companies undertaking experiments. Notably, the collaboration between bioaccess™ and Welwaze Medical Inc. for the launch of the Celbrea® medical device exemplifies how CROs can effectively navigate regulatory landscapes and market access in Colombia. Additionally, the collaboration between bioaccess™ and Caribbean Health Group seeks to position Barranquilla as a premier research destination by utilizing local resources and assistance from the Colombian Minister of Health.
With the Colombian medical devices market valued at $1.42 billion in 2022 and numerous successful experiments conducted by companies like eyeFlow and Mitralign, the region is increasingly acknowledged as a viable center for international research. According to ClinicalTrials.gov, a striking 52% of worldwide studies are now performed outside the U.S., highlighting the increasing trend of executing studies in the southern continent. Consequently, the expansion of CROss in the region is not only opportune but also offers significant Latin America CRO benefits for firms aiming to manage the intricacies of research trials.
Key Benefits of Conducting Clinical Trials in Latin America: Cost, Efficiency, and Patient Access
Carrying out research studies in the southern continent provides multiple advantages, including the Latin America CRO benefits of cost efficiency and operational effectiveness. The yearly funding in the research sector in the Andean Region has surged from $3-4 million to over $50 million, highlighting the growing dedication to investigation in this field. In contrast to the general average expenses for complete research studies in the United States, which vary from $30 to $50 million, the Latin America CRO benefits offer significant savings that can be crucial for pharmaceutical firms.
The swift patient recruitment abilities in the region contribute to Latin America CRO benefits, with recent statistics showing a recruitment rate that is 30% faster than in North America, due to its diverse population, which permits access to large treatment-naive groups. Furthermore, the presence of skilled clinical research professionals and well-established healthcare infrastructures highlights the Latin America CRO benefits, contributing to more streamlined processes. Our service capabilities encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Comprehensive reporting
These are essential for successful execution.
Patricio Ledesma, Head of Clinical Operations at Sofpromed CRO, emphasizes the importance of understanding local regulations and patient demographics to optimize study outcomes. As Julio G. Martinez-Clark, CEO of bioaccess™, aptly states, 'Colombia has recognized these benefits and has an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy.' This strategic initiative reflects a broader trend in the region, where nations are not only facilitating research studies but are also positioning themselves as influential players in the global healthcare market.
Moreover, collaborations, such as the one between bioaccess™ and Caribbean Health Group, aim to make Barranquilla a leading destination for research in Latin America, with support from Colombia's Minister of Health. The function of INVIMA, Colombia's National Food and Drug Surveillance Institute, is vital in supervising regulatory duties and ensuring adherence, thereby improving the trustworthiness of research studies. Additionally, case studies, such as the licensing framework for cannabis in Uruguay, exemplify how regulations can foster a conducive environment for innovative research.
The economic effects of medtech research studies on local economies, including job creation and healthcare enhancements, particularly emphasize the Latin America CRO benefits and the importance of carrying out experiments in this area. The worldwide expenditure on pharmaceuticals, which has risen by 35% over the last five years and is projected to grow by 38% by 2028, emphasizes the financial consequences of performing research studies in the southern continent. As these trends persist, the advantages of carrying out research studies in the region will probably draw increased interest, facilitating improved cooperation between the U.S. and regional nations.
Navigating the Regulatory Landscape: Compliance Challenges for Latin American CROs
Navigating the regulatory landscape in Latin America CRO benefits presents significant challenges for Contract Research Organizations (CROs), as each nation enforces its own unique set of regulations and approval processes. Extensive research study management services are crucial, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project oversight
- Reporting
Adherence to regional regulations, ethical principles, and global standards is crucial for the successful implementation of research studies.
This includes the critical aspect of reporting on study status, inventory, and both serious and non-serious adverse events, which are vital for maintaining transparency and accountability in the research process. As noted by Straits Research, the pharmaceutical and biopharmaceutical companies segment is the most dominant by end-users in the market, underscoring the critical role of CROs in this environment. Furthermore, with 93% of Brazil's and 85% of Argentina's population over 65 years old protected by pension systems, CROs must take into account the demographic landscape when creating studies aimed at older populations.
A strategic partnership between LEO Pharma and ICON exemplifies how the Latin America CRO benefits are enhancing clinical study execution in medical dermatology while adapting to regulatory environments. Furthermore, the research setup and approval process from ethics committees and health ministries is a crucial step that requires careful navigation to ensure compliance and expedite study initiation. With INVIMA serving as a Level 4 health authority by PAHO/WHO, maintaining current knowledge of evolving regulations and cultivating robust relationships with regulatory authorities, such as Katherine Ruiz's expertise in Regulatory Affairs for medical devices and in vitro diagnostics, are essential strategies for CROs.
These efforts not only facilitate smoother study operations but also enhance the credibility of results in the eyes of stakeholders.
Effective Patient Recruitment and Retention Strategies in Latin American Clinical Trials
Effective patient recruitment and retention strategies are essential for achieving the Latin America CRO benefits in research studies. Clinical Research Organizations (CROss) like GlobalCare Clinical Trials are increasingly utilizing localized recruitment techniques, such as community outreach and collaborations with local healthcare providers, to effectively engage potential participants. Their recent collaboration with bioaccess™ has greatly improved ambulatory services for studies in Colombia, achieving over a 50% decrease in recruitment times and a retention rate surpassing 95%.
This partnership also leverages bioaccess™ comprehensive service capabilities, including:
- Feasibility studies
- Compliance reviews
- Project management
These are essential for navigating the regulatory landscape. A recent examination of 297 studies shows that although many were randomized and blinded, only a portion focused on neglected diseases, highlighting the necessity for targeted recruitment efforts. This statistic underscores the importance of focusing on specific health issues that may otherwise be overlooked.
Furthermore, integrating digital platforms for patient engagement and education can significantly enhance awareness and interest in clinical studies. This approach aligns with the trend of employing patient-focused designs, which demonstrate a higher probability of success and quicker enrollment rates. Retention strategies are equally important; consistent communication, offering assistance throughout the process, and proactively addressing participant concerns are crucial for sustaining involvement.
As Daniel J. Herron aptly states,
The necessity of involving individuals in planning experiments and ensuring that information is accessible and understandable.
Additionally, the case study on technological advancements in medical trials illustrates how CROs are adopting predictive analytics and decentralized trials to improve trial efficiency and patient engagement. Moreover, Brazil's G20 presidency offers a unique chance to highlight the region's contributions to global health and research.
These comprehensive strategies not only improve enrollment rates but also enhance the quality of data gathered, ultimately contributing to the effectiveness and reliability of research, which highlights the Latin America CRO benefits.
The Future of CROs in Latin America: Trends and Opportunities
The landscape for Clinical Research Organizations (CROs) in Latin America is poised for significant growth, highlighting the Latin America CRO benefits driven by a confluence of emerging trends and opportunities. Providing extensive trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—CROs are equipped to tackle the unique challenges encountered by Medtech companies in the region. The early phase development services segment dominated the market in 2023, underscoring the increasing demand for innovative therapies, particularly in oncology and rare diseases, which is expected to accelerate investments in clinical research.
The Inter-American Development Bank highlights the significance of fiscal sustainability, establishing a maximum average sustainable debt level for the region at 80.5% of GDP, which is essential for fostering a stable investment climate. Furthermore, advancements in technology—such as artificial intelligence and data analytics—are transforming study design and patient recruitment processes, thereby enhancing operational efficiency. As regulatory frameworks evolve under the oversight of INVIMA, Colombia's National Food and Drug Surveillance Institute, which plays a critical role in medical device oversight and classification as a Level 4 health authority by PAHO/WHO, Latin regions are becoming an increasingly appealing destination for global research trials.
For instance, Laboratory Corporation of the United States Holdings completed the acquisition of Toxikon Corporation, reflecting strategic moves that significantly impact market dynamics. Labcorp Drug Development has capitalized on this trend, significantly enhancing its market share through strategic mergers and acquisitions in 2023. CROs that proactively adapt to these changes, while also focusing on local economic impact, will not only create jobs and stimulate economic growth but also improve healthcare outcomes, thereby contributing to the region's advancement in clinical research, highlighting the Latin America CRO benefits.
As noted by Bhushan Pawar, Assistant Manager in Healthcare, the surge in outsourcing for R&D by pharmaceutical organizations enhances efficiency and productivity, reflecting the growing recognition of Latin America CRO benefits and highlighting the necessity for comprehensive reforms in social protection systems to meet the increasing healthcare demands of an aging population.
Conclusion
The pivotal role of Contract Research Organizations (CROs) in Latin America, particularly in Colombia, underscores their significance in the evolving landscape of clinical research. By offering a comprehensive range of services—from study design to regulatory compliance—CROs not only facilitate the execution of clinical trials but also enhance operational efficiencies for pharmaceutical and biotechnology companies. The competitive advantages of conducting trials in this region, such as reduced costs, expedited regulatory processes, and a robust healthcare system, make Latin America a compelling choice for global research endeavors.
Patient recruitment and retention strategies further amplify the efficacy of clinical trials in Latin America. Utilizing localized recruitment techniques and integrating technology into patient engagement processes, CROs are successfully addressing challenges in enrollment and maintaining participant involvement. The collaboration between organizations highlights the potential for innovation and improved outcomes in clinical research, reinforcing the importance of community engagement and tailored approaches to meet specific health needs.
As the landscape continues to evolve, the future of CROs in Latin America appears promising. The growing demand for innovative therapies, coupled with advancements in technology and regulatory improvements, positions the region as an emerging hub for international clinical trials. The strategic focus on local economic impact and healthcare outcomes not only contributes to the success of clinical research but also fosters a sustainable environment for future growth. Stakeholders must recognize and harness these opportunities to navigate the complexities of clinical trials effectively, ensuring that Latin America remains a vital player on the global research stage.
Frequently Asked Questions
What are the main services provided by Contract Research Organizations (CROs) in Latin America?
CROs in Latin America offer a comprehensive suite of services including study design, site management, data collection, and regulatory compliance.
What are the benefits of conducting research studies in Colombia?
The benefits include cost savings of over 30% compared to North America and Western Europe, expedited regulatory review processes (typically 90-120 days), and access to a high-quality healthcare system.
How does the population of Colombia facilitate research studies?
Colombia has a large and diverse population of over 50 million, with about 95% having universal healthcare, which facilitates effective patient recruitment for studies.
What financial incentives are available for research and development in Colombia?
Colombia offers R&D tax incentives, including a 100% tax deduction and a 25% tax discount for investments in science, technology, and innovation.
How has the medical devices market in Colombia been characterized?
The Colombian medical devices market was valued at $1.42 billion in 2022, indicating its viability as a center for international research.
What is the significance of the recruitment rate for clinical trials in Latin America?
The recruitment rate in Latin America is 30% faster than in North America, which is beneficial for conducting studies due to the access to large treatment-naive groups.
What role does INVIMA play in research studies in Colombia?
INVIMA, Colombia's National Food and Drug Surveillance Institute, supervises regulatory duties and ensures adherence to standards, improving the trustworthiness of research studies.
How do collaborations enhance research capabilities in Colombia?
Collaborations, such as between bioaccess™ and Caribbean Health Group, aim to position Barranquilla as a leading research destination, utilizing local resources and government support.
What are the economic impacts of conducting research studies in Latin America?
Medtech research studies contribute to local economies through job creation and healthcare enhancements, highlighting the importance of conducting experiments in the region.
What trend is observed in global research studies in relation to Latin America?
A significant 52% of worldwide studies are now performed outside the U.S., indicating a growing trend of conducting research studies in Latin America.




