Overview
The benefits of Latin American clinical research include a diverse patient population, favorable regulatory conditions, lower operational costs, and improving research infrastructure, making the region attractive for pharmaceutical studies. The article supports this by detailing how these factors enhance drug efficacy evaluations and expedite study approvals, ultimately leading to faster market introductions of new therapies.
Introduction
Latin America stands at the forefront of clinical research, offering a unique blend of advantages that enhance the efficiency and effectiveness of clinical trials. With its diverse patient populations and streamlined regulatory frameworks, the region presents a compelling case for pharmaceutical companies seeking to conduct research in a dynamic environment. Recent collaborations, such as those between bioaccess™ and local health organizations, underscore the region's commitment to becoming a leading destination for clinical trials.
As stakeholders navigate the complexities of this landscape, understanding the regulatory nuances, cultural considerations, and logistical challenges becomes essential for optimizing research outcomes. This article delves into the multifaceted advantages and challenges of conducting clinical research in Latin America, highlighting its potential to shape the future of global clinical trials.
Unique Advantages of Clinical Research in Latin America
The area of South America provides a variety of distinct benefits of Latin American clinical research, greatly improving both the efficiency and effectiveness of medical assessments. The region's diverse patient population, characterized by a wide array of genetic backgrounds, is essential for evaluating drug efficacy and safety across varying demographics. This genetic variety enables more comprehensive data gathering and examination, resulting in well-informed decisions concerning treatment results.
In a significant advancement, bioaccess™ and the Caribbean Health Group have joined forces to establish Barranquilla as a premier location for research studies in South America. Backed by Colombia's Minister of Health, this initiative seeks to draw more research projects to the city, utilizing the region's advantageous conditions for studies. bioaccess™ provides extensive services, such as:
- Feasibility studies
- Site selection
- Compliance assessments
- Setup for experiments
- Import permits
- Project management
- Reporting
These services are crucial for successful execution of studies.
Additionally, many countries in South America have embraced simplified regulatory procedures that encourage faster approval and commencement of research studies. For instance, Brazil and Mexico have developed regulatory frameworks that enable faster ethical approvals compared to those in other regions. Such efficiencies can dramatically shorten the timeline from study conception to patient recruitment, allowing sponsors to introduce new therapies to the market more swiftly.
The economic landscape for research in Latin America is also beneficial, as the costs are usually lower than those in North America and Europe due to reduced operational and labor expenses. This cost-effectiveness allows pharmaceutical firms to carry out extensive research without facing the prohibitive costs often linked to studies in more developed markets. Significantly, the partnership between GlobalCare Clinical Trials and bioaccess™ has resulted in a remarkable decrease in recruitment time by over 50% and an impressive 95% retention rate, highlighting the potential for optimized study execution in this area.
Furthermore, the area is experiencing a growing infrastructure for medical studies, marked by an increasing number of trained professionals and well-equipped institutions. This development encourages the recruitment of qualified personnel and paves the way for partnerships with local hospitals and clinics, which are essential for the successful implementation of studies. Ajay Singh, senior associate dean for postgraduate medical education at Harvard Medical School, highlights the significance of offering suitable incentives for prospective study participants to promote the recruitment of a varied population.
In conclusion, the benefits of Latin American clinical research arise from its diverse patient groups, favorable regulatory landscapes, reduced operational expenses, and improving research infrastructure. Together, these elements, along with strategic partnerships such as that of bioaccess™ and Caribbean Health Group, establish the region as an attractive option for sponsors looking to enhance their trials.
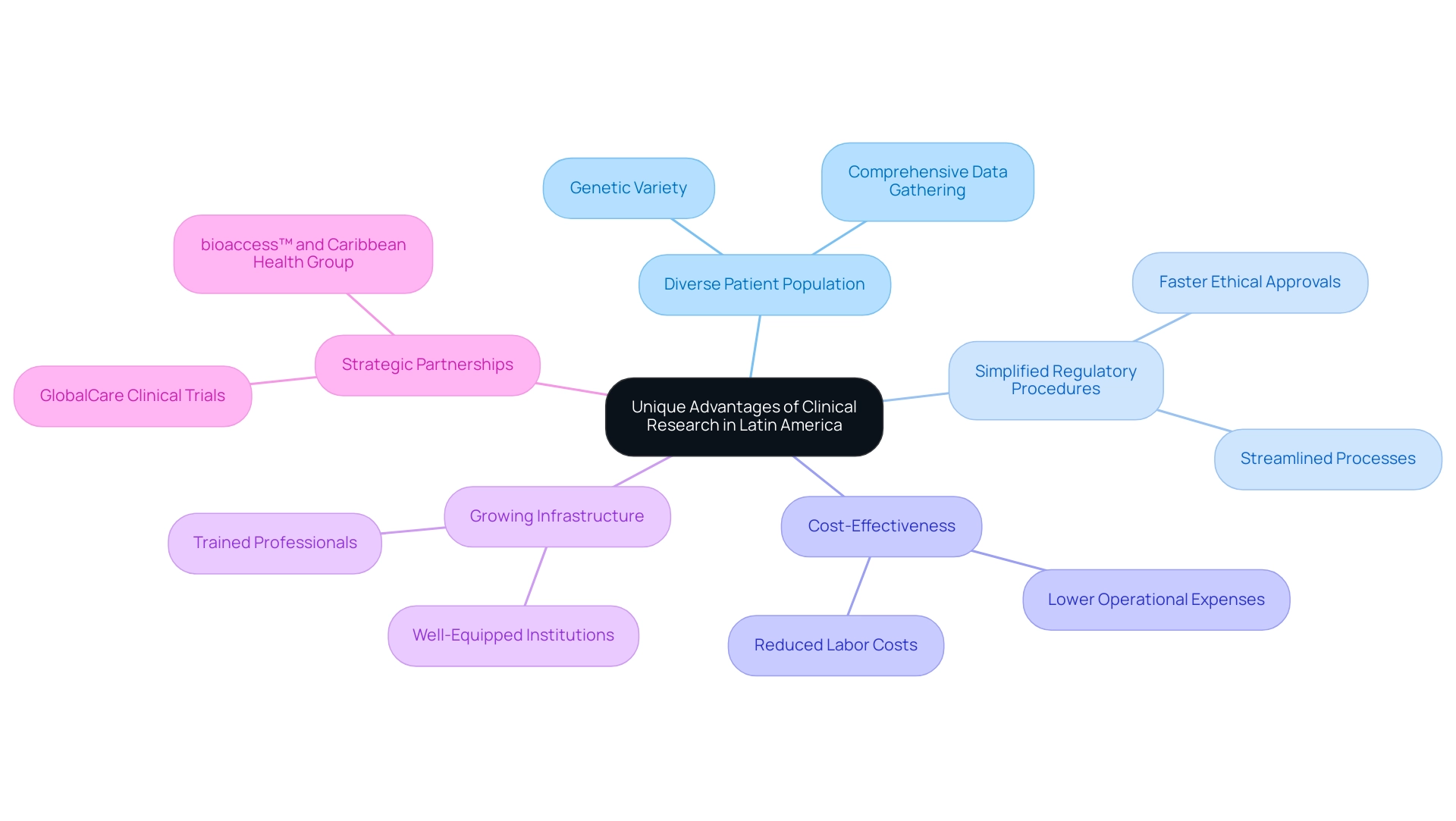
Challenges and Considerations in Latin American Clinical Trials
While the benefits of Latin American clinical research are significant for research studies, stakeholders must navigate various challenges that can influence the overall success of their initiatives. Our comprehensive clinical study management services address these hurdles, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Project management
- Support with import permits and nationalization of investigational devices
A primary concern is the significant variability in regulatory environments across the region, especially in Colombia, where INVIMA oversees medical device regulation and is recognized as a Level 4 health authority by PAHO/WHO.
At present, only 30% of the region's nations adhere to the World Health Organization's standards for air quality, with some having intricate and lengthy approval processes that can result in delays in starting trials. Understanding these regulatory landscapes is essential for effective planning and compliance with local laws.
Cultural differences also play a critical role in patient recruitment and retention. Engaging with potential participants requires a deep appreciation of local customs, beliefs, and healthcare practices. As Katherine Ruiz, a specialist in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, emphasizes, misalignment between research protocols and local practices can hinder recruitment efforts, resulting in lower participation rates or increased dropout instances, ultimately jeopardizing the integrity of the study.
Logistical challenges present another layer of complexity. Issues such as the transportation of investigational products and the management of supply chains across borders can complicate study execution. Our services encompass assistance with import permits and nationalization of investigational devices, tackling these logistical challenges with extra resources and meticulous coordination to ensure seamless operations.
Furthermore, while the cost advantages of carrying out studies in this region reflect the benefits of Latin American clinical research, sponsors must remain vigilant regarding data quality. Adherence to Good Clinical Practice (GCP) guidelines is paramount for ensuring the integrity of collected data. This may require enhanced training and oversight, which could counteract some of the initial cost advantages.
Our reporting services, which encompass detailed study status updates and monitoring of serious and non-serious adverse events, are essential for maintaining data quality and compliance throughout the research process.
The case study titled 'Air Quality and Health Risks' illustrates the environmental risks posed by non-compliance with WHO guidelines, emphasizing the need for improved air quality management in the region. This poses significant implications for research trials, as environmental factors can affect participant health and data integrity.
In summary, while this area offers a wealth of opportunities for healthcare studies, stakeholders must remain aware of the challenges posed by regulatory variability, cultural considerations, logistical issues, and data quality concerns. A nuanced understanding of these factors, along with our expert services, is crucial for effectively navigating the healthcare landscape in this dynamic region.
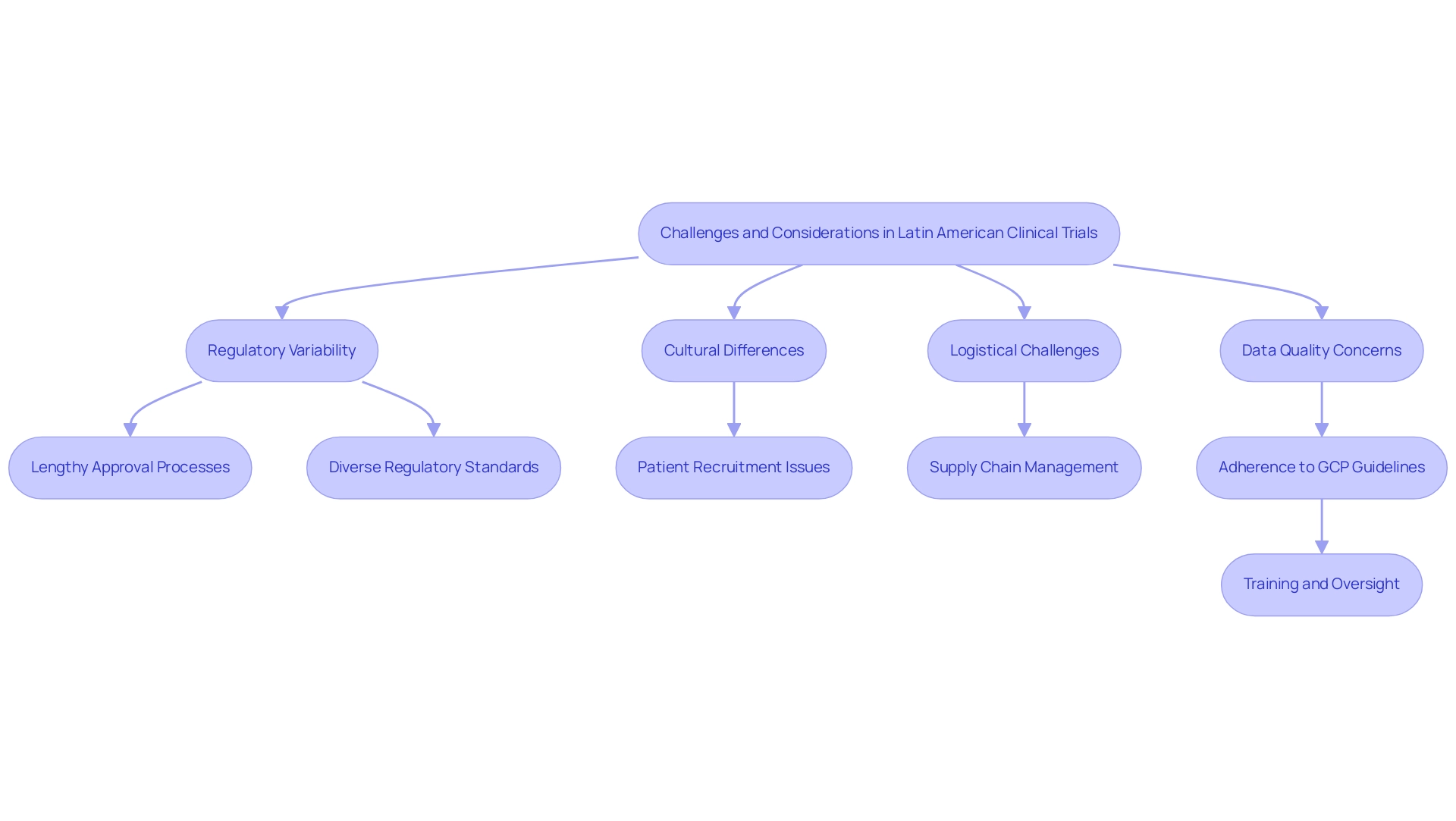
Regulatory Landscape in Latin American Clinical Research
The regulatory environment for medical research in Latin America is notably complex, but the benefits of Latin American clinical research can be significant, characterized by important variations across countries. Each country has set up its own rules and authorities that supervise medical studies. In Brazil, the National Health Surveillance Agency (ANVISA) acts as a crucial authority for the approval of research study applications, whereas in Mexico, this function is carried out by the Federal Commission for the Protection against Sanitary Risk (COFEPRIS).
Grasping the subtleties of these regulatory structures is vital for sponsors and researchers, as they directly affect the schedule and practicality of studies in their respective areas.
Colombia, in particular, showcases the benefits of Latin American clinical research by providing a compelling case for first-in-human studies due to its competitive advantages. The country offers substantial cost savings—over 30% compared to trials in North America or Western Europe—along with expedited regulatory processes, highlighting the benefits of Latin American clinical research, as the total IRB/EC and MoH (INVIMA) review can be completed in just 90-120 days. Furthermore, the World Health Organization ranks Colombia's healthcare system highly, highlighting the benefits of Latin American clinical research, as hospitals are only allowed to conduct medical studies after passing rigorous ICH/GCP certification, ensuring high-quality standards.
The patient recruitment process in Colombia showcases the benefits of Latin American clinical research, as it has a population exceeding 50 million, with about 95% covered by universal healthcare, enhancing access to diverse participant pools. Additionally, the benefits of Latin American clinical research are evident in Colombia's R&D tax incentives that can greatly assist medical device firms, including 100% tax deductions and up to $10 million in government grants for eligible projects.
Ethical considerations are crucial in the context of trials. Special ethical considerations are required when conducting studies involving indigenous peoples in Brazil, ensuring their well-being and cultural integrity. Ethics committees play a crucial role in assessing the ethical aspects of research studies, ensuring that investigations prioritize participant safety and rights.
Engaging with these committees at the outset of the planning process is essential for mitigating potential delays and enhancing the quality of the endeavor. For instance, in multicenter research, the coordinating center's ethics committee must review the protocol before it is forwarded to CONEP, which ensures a coordinated and comprehensive review process across participating institutions.
To facilitate the clinical research process in Colombia, our comprehensive clinical research management services include feasibility studies, setup, project management, and reporting. We help in navigating the regulatory landscape by ensuring compliance with local regulations, including obtaining necessary approvals from IRB/EC and INVIMA, which are essential steps in the setup process. Overall, the benefits of Latin American clinical research highlight the importance of successfully navigating the regulatory landscape in South America, which demands a comprehensive understanding of local regulations, ethical considerations, and the ability to adapt to the diverse requirements that exist across different countries.
This knowledge is essential for guaranteeing adherence and promoting successful medical studies, especially in Colombia, where the framework for health investigations is both strong and encouraging of innovation.
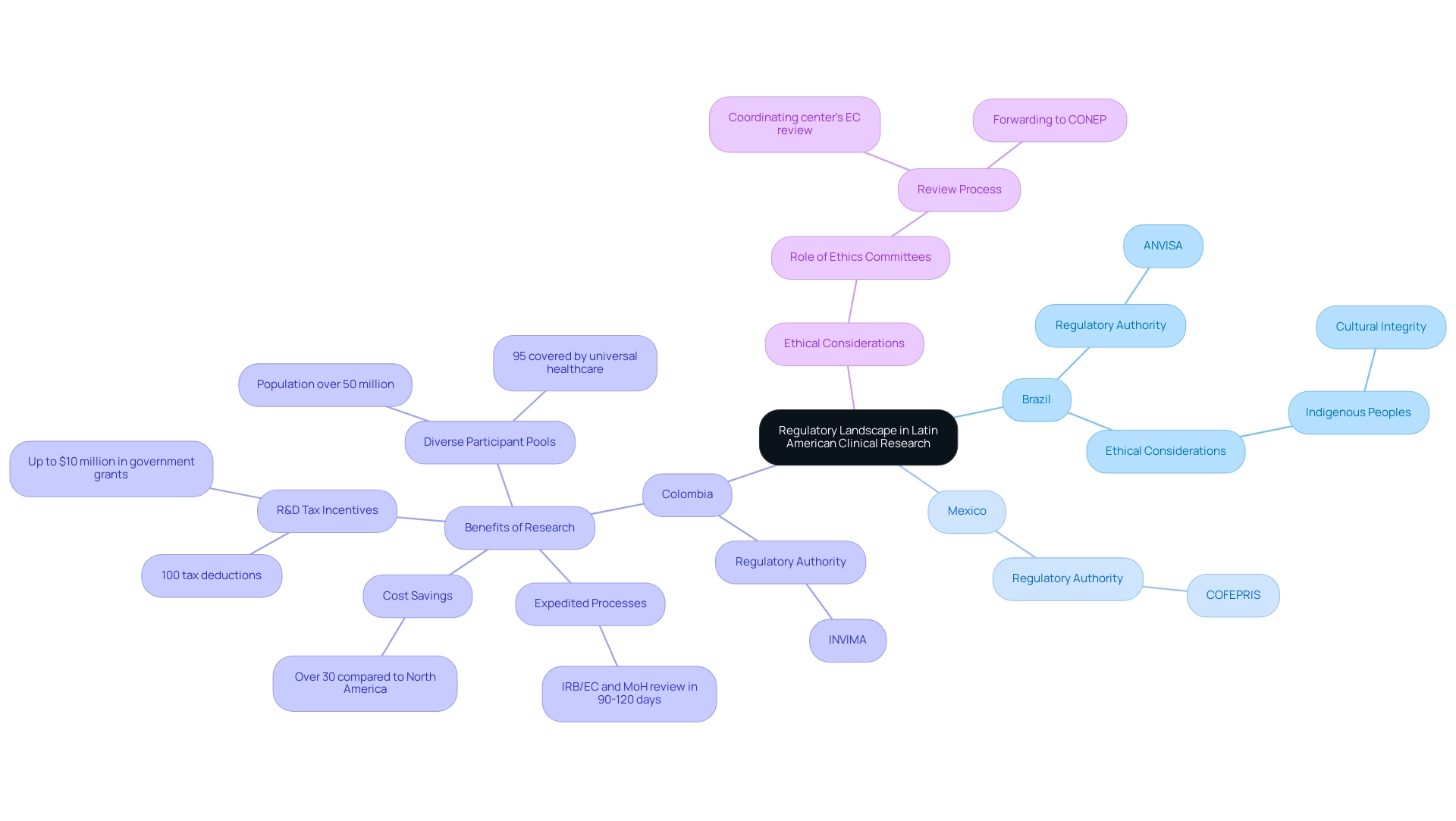
The Importance of Patient Recruitment and Retention
Patient recruitment and retention are essential components for the success of clinical studies in Latin America, particularly in Colombia, which demonstrates the benefits of Latin American clinical research, as highlighted by media coverage from outlets like Clinical Leader. For example, Clinical Leader has reported on the growing number of scientific publications in oncology, emphasizing the significance of these studies in tackling health challenges and enhancing healthcare outcomes. Effectively engaging patients not only facilitates timely study completion but also enhances the integrity of the data collected.
Researchers must implement targeted outreach strategies that resonate with the local population, which may involve:
- Collaborating with community leaders
- Leveraging social media platforms
- Providing educational resources about the clinical research process
Retention strategies are equally crucial, as elevated dropout rates can compromise study outcomes. Clear and transparent communication with participants is vital; ensuring they comprehend the study's purpose and their role within it can significantly influence their commitment. Ongoing support throughout the study, including regular check-ins and addressing concerns, fosters participant engagement.
Cultural sensitivity is also critical in enhancing patient retention. A thorough understanding and respect for local customs and values can build trust and encourage participants to stay engaged in the study. For instance, providing culturally pertinent incentives or recognizing local health beliefs can greatly improve participant satisfaction and dedication to the study.
As mentioned by Gustavo Werutsky, 'Pharmaceutical companies have financed most oncology studies in South America and the Caribbean, and additional funding will also be required from government or private foundations.' This underscores the necessity for enhanced support in patient recruitment and retention efforts to drive economic growth and job creation in the region. The effect of medical trials on local economies is significant, as they not only generate employment within the healthcare field but also aid overall economic development through enhanced healthcare results and increased funding in studies.
The case analysis titled 'Challenges and Opportunities for Medical Studies in the region' demonstrates that despite obstacles such as regulatory timelines and limited financial resources, the area has competitive advantages, including a high willingness of patients to engage in trials and a varied population.
The benefits of Latin American clinical research underscore the significance of patient recruitment and retention strategies, which are crucial in ensuring the dependability and success of medical studies in the region. By prioritizing engagement, fostering open communication, and embracing cultural sensitivity, investigators can enhance study outcomes, achieve their objectives, and contribute positively to local economies.
Future Trends in Latin American Clinical Research
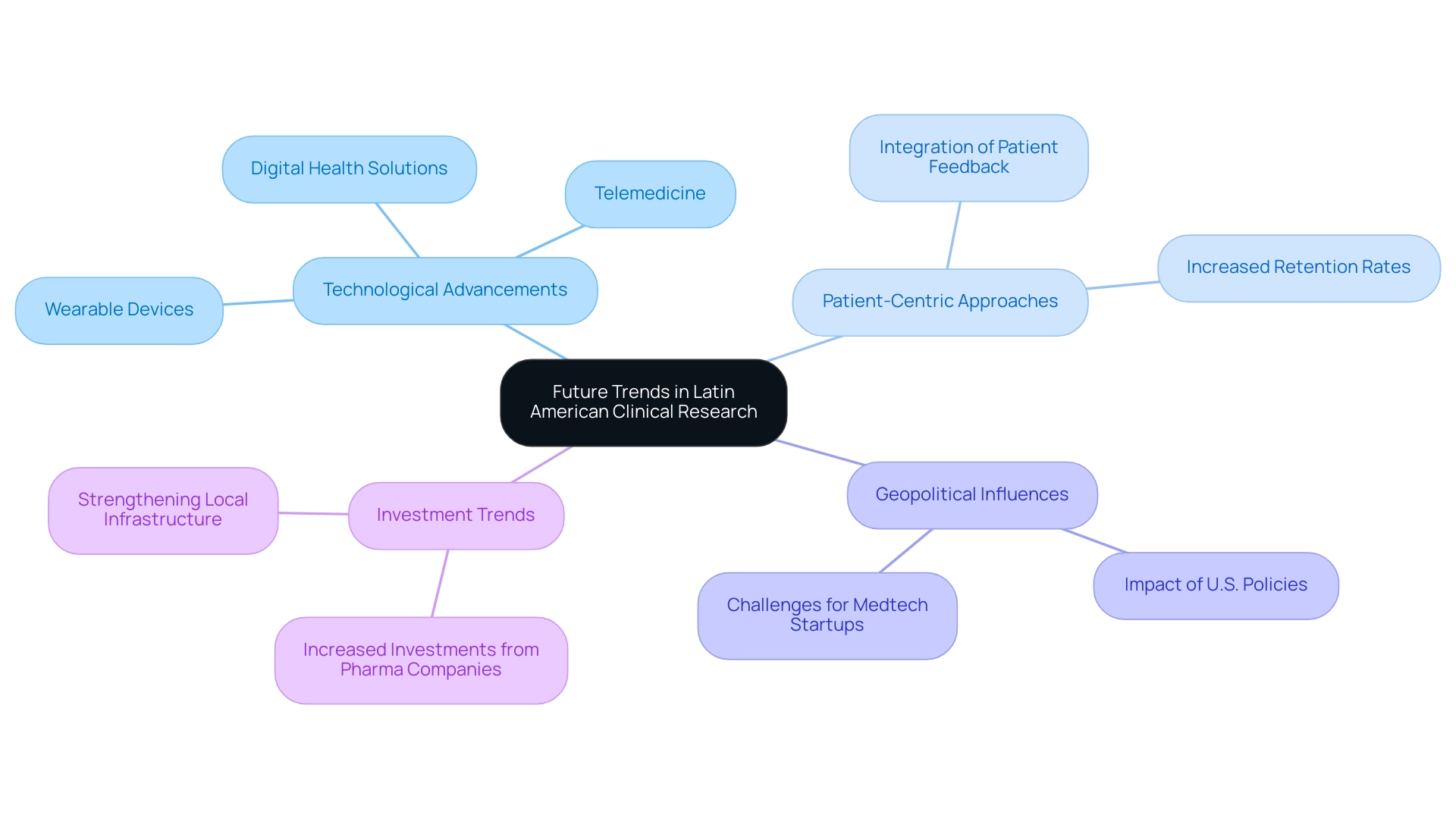
The terrain of medical research in South America is experiencing substantial change, propelled by technological progress, regulatory adjustments, and evolving patient demographics. Notably, the proportion of individuals using technology to engage with the healthcare system surged from 12.5% in 2011 to 27.4% in 2018, reflecting a growing trend towards digital health solutions. Technologies such as telemedicine and wearable devices are becoming integral, enhancing patient monitoring and data collection capabilities.
These innovations not only simplify testing processes but also encourage remote patient involvement, effectively expanding the scope of medical research. However, this advancement encounters challenges, especially considering geopolitical factors and particular obstacles faced by Medtech startups. Regulatory complexities, language barriers, and fragmentation of resources obstruct effective cooperation between South American hospitals and American research clients.
President Trump's executive orders have threatened global health initiatives that depend on the United States, potentially affecting joint efforts in research across Latin America. Such geopolitical dynamics emphasize the necessity for resilience and innovation in the region's health systems.
Moreover, there is a pronounced shift towards patient-centric methods in clinical studies. Researchers increasingly acknowledge the value of integrating patient feedback into the design and execution of studies. For example, a recent study indicated that experiments incorporating patient insights saw a 30% increase in retention rates.
This focus is expected to enhance participant satisfaction and retention rates, ultimately promoting more dependable study results. As Carlos Javier Regazzoni, Director of the Human Security and Global Health Committee at the Argentine Council on Foreign Relations, aptly states,
- "Achieving a healthy society requires communities to rise above selfish and partisan interests and commit to collective action."
The benefits of Latin American clinical research have led to the region becoming a strategic center for multinational pharmaceutical companies that aim to carry out studies within diverse populations in response to the increasing need for innovative therapies.
This trend is poised to stimulate investments in local investigative infrastructure, which will ultimately bolster the benefits of Latin American clinical research and strengthen the region's capacity for high-quality medical studies. Services such as feasibility studies, site selection, compliance reviews, and setup are crucial in tackling the challenges encountered by Medtech firms, enabling smoother implementation of research studies. Moreover, the partnership between Greenlight Guru and bioaccess™ to expedite Medtech advancements and studies in South America emphasizes the dedication to closing the divides in medical exploration and promoting global collaborations.
Additionally, AI and Big Data are being investigated as instruments to tackle intricate social challenges, such as healthcare delivery, suggesting their possible contribution to improving medical study methodologies.
In summary, the future of medical studies in South America showcases the benefits of Latin American clinical research, characterized by the incorporation of digital health technologies, a dedication to patient-focused methodologies, and a surge of investments from international pharmaceutical companies. These developments promise to enhance both the efficiency and effectiveness of clinical trials, showcasing the benefits of Latin American clinical research and positioning the region as a pivotal player in the global clinical research arena.
Conclusion
Latin America offers a promising landscape for clinical research, characterized by its diverse patient populations, streamlined regulatory processes, and favorable economic conditions. The region's genetic diversity enhances the evaluation of drug efficacy and safety, while strategic collaborations like that of bioaccess™ and local health organizations solidify its position as a leading destination for clinical trials. Moreover, the economic advantages of conducting trials in Latin America, including lower operational costs, are significant, allowing for extensive research without the financial burdens often faced in more developed markets.
However, navigating this dynamic environment is not without its challenges. Variability in regulatory frameworks across countries, cultural considerations in patient engagement, and logistical hurdles must be addressed to optimize research outcomes. Understanding these complexities is essential for stakeholders aiming to successfully execute clinical trials. The importance of patient recruitment and retention cannot be overstated, as these elements are crucial for ensuring reliable data and achieving research objectives.
Looking to the future, technological advancements and a shift towards patient-centric approaches are set to transform the clinical research landscape in Latin America. The integration of digital health solutions and the increasing importance of incorporating patient feedback will enhance trial efficiency and participant satisfaction. As multinational pharmaceutical companies continue to invest in the region, Latin America is poised to become a pivotal player in the global clinical research arena, shaping the future of healthcare innovation while contributing to local economic growth.
Frequently Asked Questions
What are the key benefits of conducting clinical research in South America?
The key benefits include a diverse patient population with various genetic backgrounds, simplified regulatory procedures for faster approvals, lower operational costs compared to North America and Europe, and an improving infrastructure with more trained professionals and equipped institutions.
How does the diversity of the patient population in South America impact clinical research?
The diverse patient population allows for comprehensive data gathering and examination, which is essential for evaluating drug efficacy and safety across different demographics.
What services does bioaccess™ provide to enhance clinical research in South America?
bioaccess™ offers services such as feasibility studies, site selection, compliance assessments, setup for experiments, import permits, project management, and reporting.
How have regulatory frameworks in countries like Brazil and Mexico improved clinical research timelines?
These countries have developed regulatory frameworks that enable faster ethical approvals, significantly shortening the timeline from study conception to patient recruitment.
What is the economic advantage of conducting research in Latin America?
The costs of conducting research in Latin America are generally lower than in North America and Europe due to reduced operational and labor expenses, allowing for extensive research without prohibitive costs.
What challenges do stakeholders face when conducting clinical research in South America?
Stakeholders must navigate regulatory variability, cultural differences affecting patient recruitment, logistical challenges related to product transportation, and the need to maintain data quality.
Why is understanding local customs and healthcare practices important in patient recruitment?
Misalignment between research protocols and local practices can hinder recruitment efforts, leading to lower participation rates or increased dropout instances, which jeopardizes the integrity of the study.
How does the quality of data collected impact the advantages of conducting research in Latin America?
While cost advantages exist, adherence to Good Clinical Practice (GCP) guidelines is essential for ensuring data integrity, which may require additional training and oversight, potentially offsetting initial cost benefits.
What role does the environmental quality play in clinical research in South America?
Poor air quality and non-compliance with WHO guidelines can pose health risks to participants and affect the integrity of data collected during research trials.
What is the significance of partnerships, such as that between GlobalCare Clinical Trials and bioaccess™?
Such partnerships can lead to improved study execution, as evidenced by reduced recruitment times and high retention rates, making the region more attractive for clinical trials.




