Overview
The key considerations for First-in-Human (FIH) studies include assessing safety profiles, establishing appropriate dosing regimens, and navigating ethical and regulatory frameworks to ensure participant well-being and data integrity. The article emphasizes that a rigorous approach to these considerations, such as the careful selection of participants and robust monitoring of adverse events, is essential for balancing innovation in clinical research with the protection of human subjects.
Introduction
First-in-Human (FIH) studies represent a pivotal moment in the realm of clinical research, where innovative therapies are first introduced to human subjects. These trials are not only essential for assessing the safety and efficacy of new treatments but also require meticulous planning and ethical considerations. As the landscape of medical research evolves, understanding the intricacies of FIH studies becomes increasingly critical for researchers, sponsors, and regulatory bodies.
This article delves into the fundamental aspects of FIH trials, exploring key factors such as:
- Dose selection
- Participant recruitment
- Regulatory compliance
- The imperative of monitoring adverse events
Through a comprehensive examination of these elements, the discussion aims to illuminate the complexities and responsibilities inherent in conducting successful FIH studies, ultimately contributing to the advancement of medical science and patient care.
Understanding the Fundamentals of First-in-Human Studies
Key considerations for First-in-Human (FIH) studies represent a critical juncture in clinical research, marking the initial phase where investigational drugs or therapies are administered to human subjects for the first time. These investigations are crucial for assessing the security, tolerability, and pharmacokinetics of innovative therapies. Key Considerations for First-in-Human (FIH) Studies are characterized by their stringent design and adherence to ethical standards, often involving the navigation of unknown risks and necessitating a careful balance between innovation and participant well-being.
Significant instances involve:
- Tioga Cardiovascular's revolutionary Luna™ TMVR System, which is presently in its initial human testing phase
- Avantec Vascular's cutting-edge vascular device research in Latin America, backed by bioaccess™, which plays a crucial part in streamlining the research's regulatory procedures and investigator selection
Key Considerations for First-in-Human (FIH) studies include:
- Assessing safety profiles
- Establishing appropriate dosing regimens
- Collecting preliminary efficacy data
Recent statistics indicate that the success rate of medical experiments increased by 11.3 percentage points when non-industrial partners participated, underscoring the value of collaborative efforts in enhancing study outcomes.
This trend is illustrated by the successful first-in-human implantations of PAVmed's PortIO™ Intraosseous Infusion System in Colombia, which showed positive results in medical evaluations. Furthermore, a case analysis comparing the success factors in Phase 1 evaluations for New Molecular Entities (NME) and Biologicals reveals that while experience remains a critical factor, the success ratios for Biologicals were significantly higher than for Names, emphasizing the differences in outcomes based on drug type. This information is essential for grasping the complexities of clinical studies, particularly in the evolving landscape of early-feasibility research in areas like Latin America, and should be taken into account by researchers, sponsors, and regulatory bodies involved in these efforts.
Key Considerations for Starting Dose Selection in FIH Trials
Selecting the starting dose in first-in-human (FIH) trials requires careful attention to the key considerations for first-in-human (FIH) studies, as it presents a nuanced challenge that necessitates a thorough evaluation of various factors, including:
- Preclinical data
- Pharmacodynamics
- Pharmacokinetics
The main goal is to determine a dose that optimally balances therapeutic efficacy and security, as these are key considerations for first-in-human (FIH) studies, thereby maximizing potential benefits while minimizing risks. A common approach is to reference the Maximum Tolerated Dose (MTD) established in animal studies, although many researchers advocate for a more conservative stance through allometric scaling techniques.
Furthermore, the implementation of robust safety monitoring protocols is essential to promptly adjust dosing in response to observed adverse events. This meticulous approach to calibrating starting doses is critical in protecting participant health and upholding the integrity of the trial, highlighting the key considerations for First-in-Human (FIH) studies. Recent research indicates that the total count of MTD determinations across different categories reached 79, emphasizing the importance of systematically derived dosing strategies in medical research.
Notably, the choice of MRSD determination method varied significantly by publication year, emphasizing the evolving nature of these methodologies. The statistical methods employed in these MTD determinations included:
- PK model-based
- PD model-based
- PK–PD model-based approaches
These contribute to a more nuanced understanding of dose selection. As mentioned by Dr. In-Jin Jang, insightful comments and suggestions can refine these processes, contributing to enhanced results in early feasibility assessments.
The results from case analyses, particularly in the field of monoclonal antibodies, underscore the Key Considerations for First-in-Human (FIH) Studies, demonstrating that effective dose selection can significantly enhance the clinical development process by providing clearer insights into effectiveness and efficacy outcomes.
Choosing the Right Participants: Healthy Volunteers vs. Patients
The recruitment strategy in First-in-Human (FIH) studies—whether to involve healthy volunteers or patients—is one of the key considerations for First-in-Human (FIH) studies that must be closely aligned with the study's objectives. Healthy participants are usually chosen for early-phase studies to evaluate security and pharmacokinetics while reducing the confounding variables linked to existing illnesses. However, when the drug's intended use targets specific conditions, involving patients who exhibit those conditions may provide more relevant data.
This choice inherently raises ethical considerations that are among the Key Considerations for First-in-Human (FIH) Studies, particularly concerning the risks posed to vulnerable populations. Researchers encounter the challenge of balancing rigorous scientific objectives with their ethical duties, which includes key considerations for First-in-Human (FIH) studies to ensure that informed consent is comprehensively acquired and participant well-being is prioritized throughout the study. To enhance safety in these early-phase studies, the practice of sentinel dosing is recommended, where subjects are dosed sequentially with observation periods, allowing researchers to monitor safety more effectively.
Additionally, recent news highlights concerns regarding stopping rules in protocols, which allowed drug-related severe adverse events to occur in half of the healthy volunteers without necessitating a pause in dose escalation. This underscores the ethical implications and risks involved in participant selection. Moreover, recent statistics reveal that 77.3% of non-white individuals deem communication with the oversight—whether the hospital or the sponsoring company—to be important, in contrast to 73.4% of their white, non-Hispanic counterparts.
This highlights the necessity for transparency and trust in the recruitment process, linking back to the ethical responsibilities researchers must uphold. As bioaccess® illustrates through its extensive trial management services—including feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and reporting—ensuring participant awareness of potential risks is vital in promoting a responsible research environment. With over 20 years of experience in Medtech, bioaccess® is skilled at managing various projects, including Early-Feasibility Evaluations (EFS), Pilot Trials, and Post-Market Clinical Follow-Up Assessments (PMCF), ensuring that transparency and trust are at the forefront of their recruitment process.
Navigating Regulatory and Ethical Considerations in FIH Studies
Navigating the regulatory and ethical landscape of First-in-Human (FIH) trials involves key considerations for First-in-Human (FIH) studies, which are essential for ensuring participant safety and the integrity of research. Guidelines from prominent regulatory bodies, including the FDA, EMA, and ICH, lay the groundwork for study design, informed consent protocols, risk assessment processes, and ongoing monitoring. Regulation (EU) No. 536/2014, as expressed by the EMA, highlights the significance of openness in studies carried out within the European Union, promoting the disclosure of study results to boost public confidence. As stated by the EMA, this regulation provides a legal basis for the release of clinical study results, emphasizing the need for accountability in clinical research. Ethical considerations are paramount; researchers must ensure that participants are comprehensively informed of potential risks and benefits while safeguarding the objectivity of study outcomes through unbiased reporting.
Institutional Review Boards (IRBs) serve a critical function in overseeing ethical compliance, ensuring that all studies adhere to established standards. This adherence not only protects participants but also bolsters the credibility of research findings. Real-world applications of these guidelines can be seen in programs like the CATT Program and the Pre-Pre-IND Interact Program, which offer early drug-development advice for biologic products, facilitating communication with the FDA.
As investigative medical studies gain traction for earlier access to human data, particularly in situations involving limited exposure and no therapeutic intent, the need for stringent ethical oversight becomes even more pronounced. For instance, if the background rate of an adverse event is estimated at 1%, the probability of observing such an event in a cohort of six participants rises to approximately 5%, and in a cohort of ten, it reaches nearly 9%. Such statistics highlight the necessity for careful risk assessment and monitoring as part of the Key Considerations for First-in-Human (FIH) Studies, reinforcing the imperative for robust ethical frameworks.
Additionally, recent recommendations regarding eligibility criteria for including pediatric patients in cancer clinical studies further emphasize the evolving landscape of ethical oversight in clinical research. With more than 20 years of experience in Medtech, bioaccess® is prepared to handle Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, and other crucial evaluations, maneuvering through these complexities while ensuring compliance and increasing the chances of successful outcomes.
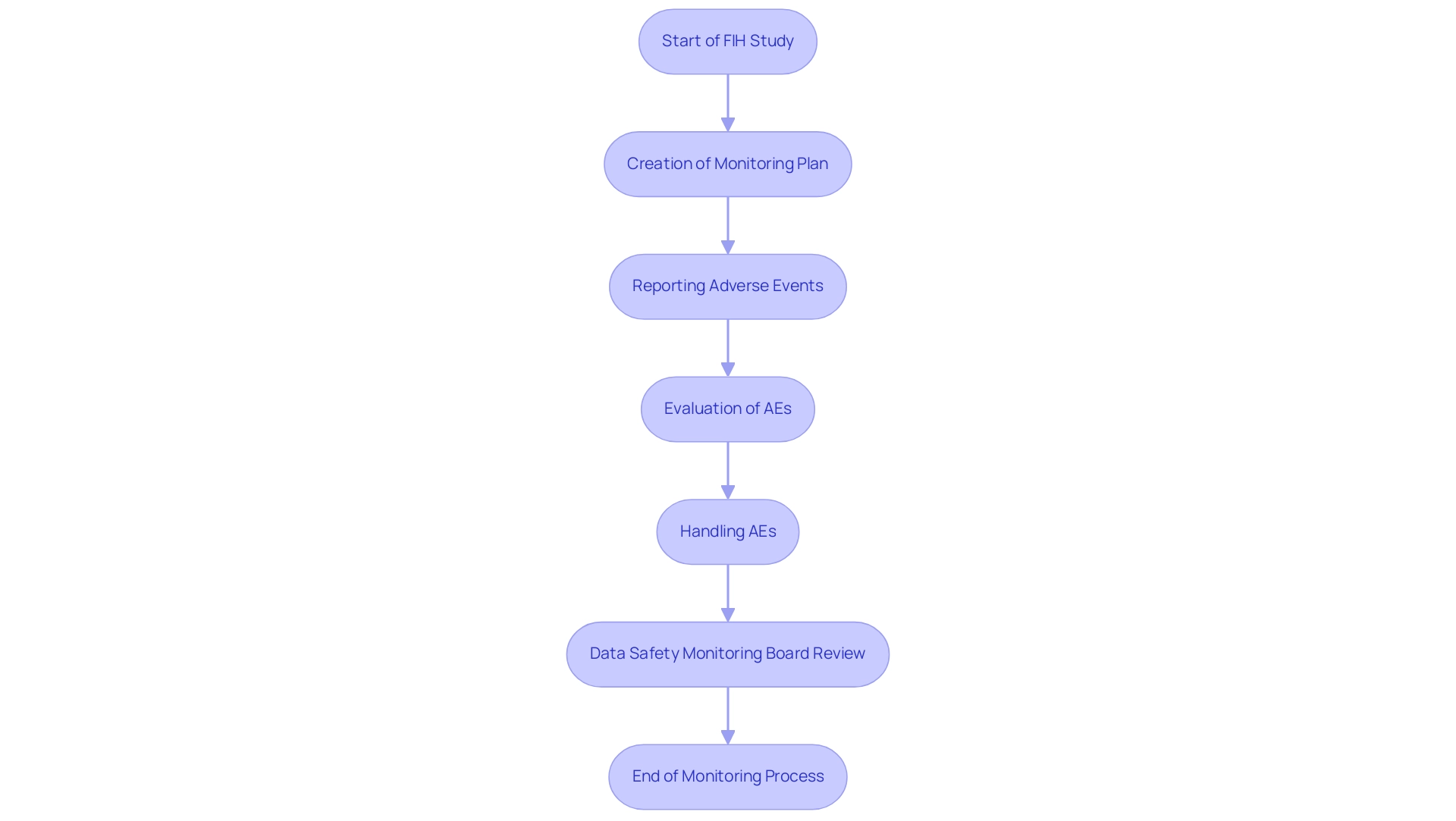
Monitoring Adverse Events: Ensuring Safety in FIH Trials
Monitoring adverse events during First-in-Human (FIH) studies is one of the key considerations for First-in-Human (FIH) studies to protect participant health and ensure the overall success of the research. At bioaccess®, our extensive clinical study management services encompass the creation of a thorough monitoring plan before the study, which details procedures for reporting, evaluating, and handling adverse events. This framework encompasses the establishment of Data Safety Monitoring Boards (DSMBs), essential for the independent evaluation of data and for offering recommendations on study continuation.
Recent findings indicate that:
- 95.8% of reported adverse events (AEs) were mild or moderate, totaling an impressive 50,570 AEs, underscoring the necessity of effective monitoring.
- Specific adverse events, such as infections, were found to be more prevalent in younger children, while psychiatric disorders were more common in middle-aged adults, highlighting the importance of tailored monitoring approaches.
The prompt recognition and handling of adverse events not only protect participants but also contribute to the ongoing assessment of the investigational product’s risk profile.
This proactive method improves decision-making in research by incorporating key considerations for First-in-Human (FIH) studies, enabling informed choices about the continuation or alteration of study protocols. As mentioned by V. Cornelius,
Statistical methods for the analysis of adverse event data in randomized controlled studies: a scoping review and taxonomy,
employing advanced statistical frameworks greatly enhances the evaluation of safety data in research. Moreover, case analysis exploring Bayesian techniques for assessing emerging adverse event data demonstrate how utilizing comparable medical occurrences can provide valuable insights into AE probabilities, emphasizing the essential role of oversight in medical research.
With over 20 years of expertise in Medtech, bioaccess® is dedicated to navigating regulatory environments effectively, ensuring your clinical studies, including Early-Feasibility Studies (EFS), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), are conducted with utmost compliance and efficiency, supported by the flexibility and specialized knowledge required for medical device clinical trials.
Conclusion
First-in-Human (FIH) studies serve as a cornerstone in the advancement of clinical research, marking the transition from preclinical assessments to human trials. This article has explored the essential components of FIH studies, including:
- Dose selection
- Participant recruitment
- Regulatory compliance
- The critical need for monitoring adverse events
Each of these elements plays a vital role in ensuring the safety and efficacy of new treatments, ultimately contributing to the broader goals of medical innovation and improved patient care.
The careful selection of starting doses, guided by preclinical data and safety monitoring protocols, is paramount to balancing therapeutic benefits with participant safety. Furthermore, the decision to recruit healthy volunteers versus patients requires a nuanced approach, weighing scientific objectives against ethical responsibilities. Regulatory guidelines provide a framework for conducting these studies, ensuring transparency and accountability while safeguarding participant welfare.
Monitoring adverse events is crucial in FIH trials, as it not only protects participants but also informs the ongoing evaluation of investigational therapies. By implementing robust safety measures and employing sophisticated statistical methods, researchers can enhance the reliability of their findings and support informed decision-making throughout the trial process.
In summary, the complexities of FIH studies demand a meticulous approach that prioritizes ethical considerations and participant safety. As the landscape of clinical research evolves, a comprehensive understanding of these critical elements will be essential for researchers, sponsors, and regulatory bodies alike, ultimately driving progress in medical science and enhancing patient outcomes.
Frequently Asked Questions
What are First-in-Human (FIH) studies?
First-in-Human (FIH) studies are the initial phase of clinical research where investigational drugs or therapies are administered to human subjects for the first time. They are essential for assessing the safety, tolerability, and pharmacokinetics of new therapies.
What are the key considerations for FIH studies?
Key considerations for FIH studies include assessing safety profiles, establishing appropriate dosing regimens, and collecting preliminary efficacy data.
Why are non-industrial partners important in FIH studies?
Recent statistics indicate that the success rate of medical experiments increased by 11.3 percentage points when non-industrial partners participated, highlighting the value of collaborative efforts in improving study outcomes.
Can you provide examples of significant FIH studies?
Significant instances include Tioga Cardiovascular's Luna™ TMVR System, which is in its initial human testing phase, and Avantec Vascular's vascular device research in Latin America, which is supported by bioaccess™ to streamline regulatory processes.
What factors are considered when selecting the starting dose in FIH trials?
Factors include preclinical data, pharmacodynamics, and pharmacokinetics, with the goal of balancing therapeutic efficacy and safety.
What is the Maximum Tolerated Dose (MTD) and its relevance in FIH studies?
The Maximum Tolerated Dose (MTD) is a reference point established in animal studies that helps researchers determine an optimal starting dose for human trials, although conservative approaches through allometric scaling are also advocated.
What is the importance of safety monitoring protocols in FIH studies?
Robust safety monitoring protocols are essential for promptly adjusting dosing in response to observed adverse events, ensuring participant health and maintaining trial integrity.
How has the methodology for MTD determination evolved?
The choice of MTD determination method has varied by publication year, with statistical methods including PK model-based, PD model-based, and PK–PD model-based approaches contributing to a better understanding of dose selection.
What role does effective dose selection play in clinical development?
Effective dose selection can significantly enhance the clinical development process by providing clearer insights into effectiveness and efficacy outcomes, particularly in the development of monoclonal antibodies.




