Introduction
The 510(k) approval process stands as a cornerstone of medical device regulation in the United States, offering a pathway for manufacturers to bring innovative products to market while ensuring patient safety. Established by the U.S. Food and Drug Administration (FDA), this process allows companies to demonstrate that their devices are substantially equivalent to existing, legally marketed devices, thus bypassing the more rigorous Premarket Approval (PMA) process reserved for higher-risk devices.
With approximately 80% of medical devices falling under the Class II category, understanding the intricacies of the 510(k) process is essential for manufacturers aiming to navigate the regulatory landscape effectively. This article delves into the nuances of the 510(k) approval process, outlining its purpose, the steps involved, and the critical concept of substantial equivalence, while also addressing the common challenges faced by manufacturers and strategies to overcome them.
Understanding 510(k) Approval: Definition and Purpose
The 510(k) authorization system, which is an example of what is 510k approval, created by the U.S. Food and Drug Administration (FDA), acts as a crucial regulatory route for medical equipment manufacturers. This method enables companies to show that their equipment is 'substantially equivalent' to an already legally marketed product, thus simplifying the path to market. What is 510k approval primarily aimed at ensuring is that new products meet safety and efficacy standards without the need for the exhaustive Premarket Approval (PMA) process, which is typically reserved for high-risk items.
It is important to note that low-risk Class I items may not require a 510(k), while most Class II items do. This oversight route is especially important, as it enables the prompt launch of innovative healthcare tools while maintaining strict patient safety standards. Recent statistics indicate that approximately 80% of all medical devices fall under the Class II category, which is governed by what is 510k approval, highlighting its critical role in the industry.
In this context, comprehensive clinical trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—are essential to navigate the complexities of the compliance landscape effectively. Specialists such as Ana Criado, Director of Regulatory Affairs with vast experience in Colombia’s regulatory landscape, play a vital role in assisting companies through these procedures. The average time to clearance for a 510(k) submission is approximately five to six months, which illustrates what is 510k approval and demonstrates the efficiency of this system.
Katrina Rogers aptly notes,
Readily available data from the FDA tells us we can expect a reasonably high (though not 100%) success rate for our PMA and 510(k) medical products submissions.
This highlights the dependability of the 510(k) system, or what is 510k approval, as an essential component in the oversight framework, allowing manufacturers to introduce their products to the market effectively while ensuring adherence to FDA guidelines. A significant milestone in this procedure is obtaining a Substantially Equivalent (SE) letter from the FDA, which permits the marketing of a product in the U.S., signifying adherence to FDA regulations and marking a considerable accomplishment for companies.
Navigating the 510(k) Approval Process: Steps and Requirements
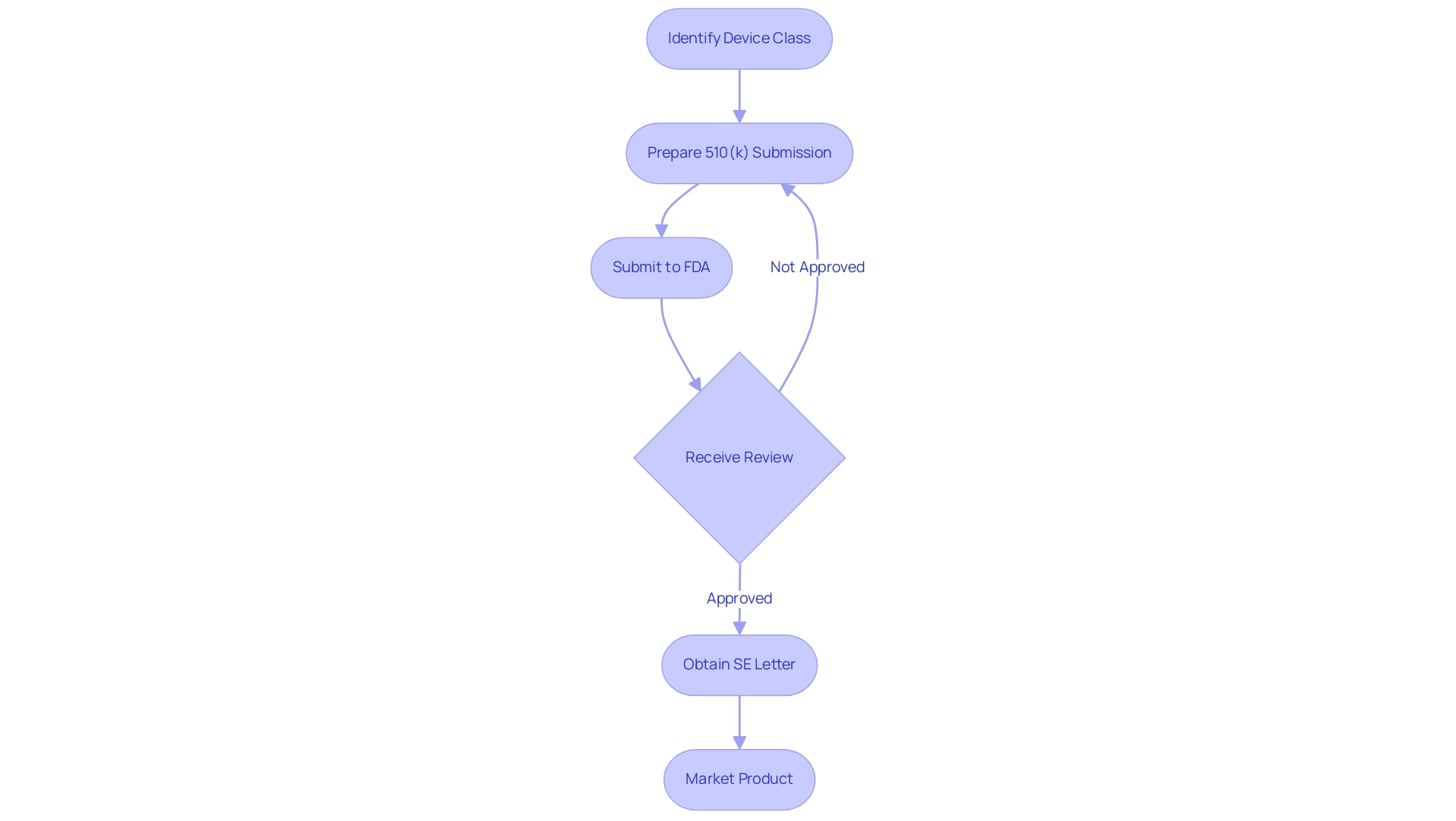
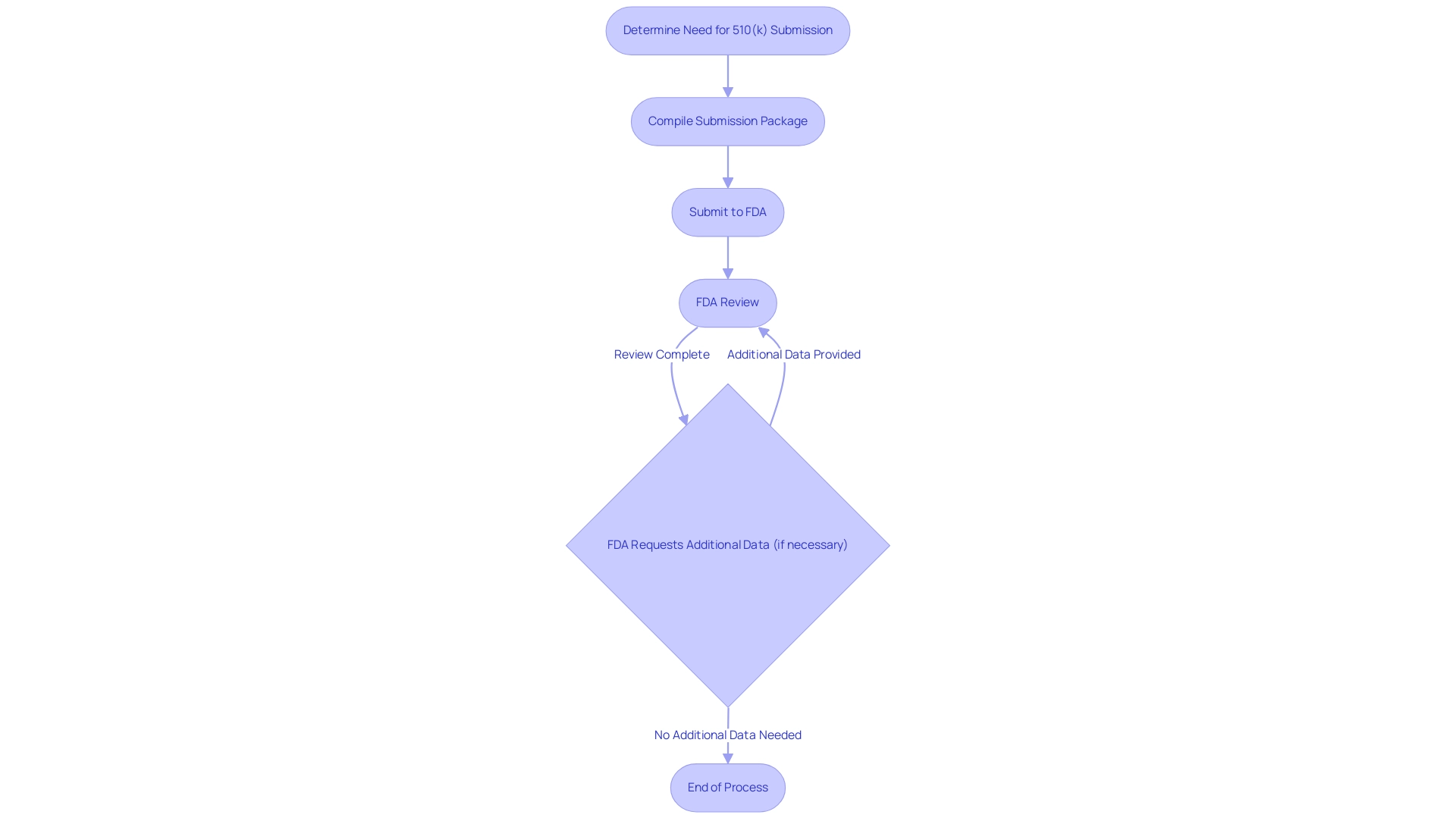
The process of what is 510k approval is a meticulous pathway that involves several essential steps, requiring a deep understanding of compliance matters. Experts such as Katherine Ruiz, a Regulatory Affairs specialist focusing on healthcare products and in vitro diagnostics in Colombia, highlight the significance of manufacturers understanding what is 510k approval to determine whether their product requires a 510(k) submission. Katherine possesses a degree in industrial microbiology from Universidad Javeriana and has extensive experience at INVIMA, where she facilitated import licenses for diagnostic reagents and medical equipment.
Upon confirmation of the need for a submission, manufacturers must compile an exhaustive package, including detailed device descriptions, intended use, and data demonstrating substantial equivalence to a predicate device. This comprehensive documentation serves as the foundation for the FDA's evaluation. Significantly, Ana Criado, Director of Affairs and a professor in biomedical engineering at Universidad Javeriana and Universidad de los Andes, plays a crucial role in guiding manufacturers through these requirements, drawing on her extensive experience with Colombia’s agency, INVIMA, and her qualifications in chemical pharmacology and health economics.
Ana utilizes different governance structures and methodologies to ensure compliance and successful navigation of the approval procedure. A significant illustration of this process is ZuriMED Technologies AG, which recently secured FDA approval for its FiberLocker® system, enabling them to improve their product range in the healthcare sector. Once the submission is forwarded to the FDA, the agency conducts a thorough review and may request further data or clarifications to ensure compliance with regulatory standards.
Recent enhancements in the average approval time for panel-track supplements, with reports indicating a median of 304 days in the first half of 2023—a 27% reduction compared to 2022 levels, according to BTIG analysts—highlight the evolving landscape of medical technology regulation. While the usual length for the entire process, known as what is 510k approval, hovers around 90 days, it can vary significantly based on the complexity of the product and the completeness of the submission. This underscores the necessity for manufacturers to stay informed about recent changes, such as the FDA's withdrawal of recognition for Accelerated Device Approval Services, LLC, in March 2021, emphasizing the importance of adhering to the latest regulatory requirements.
The Importance of Substantial Equivalence in 510(k) Submissions
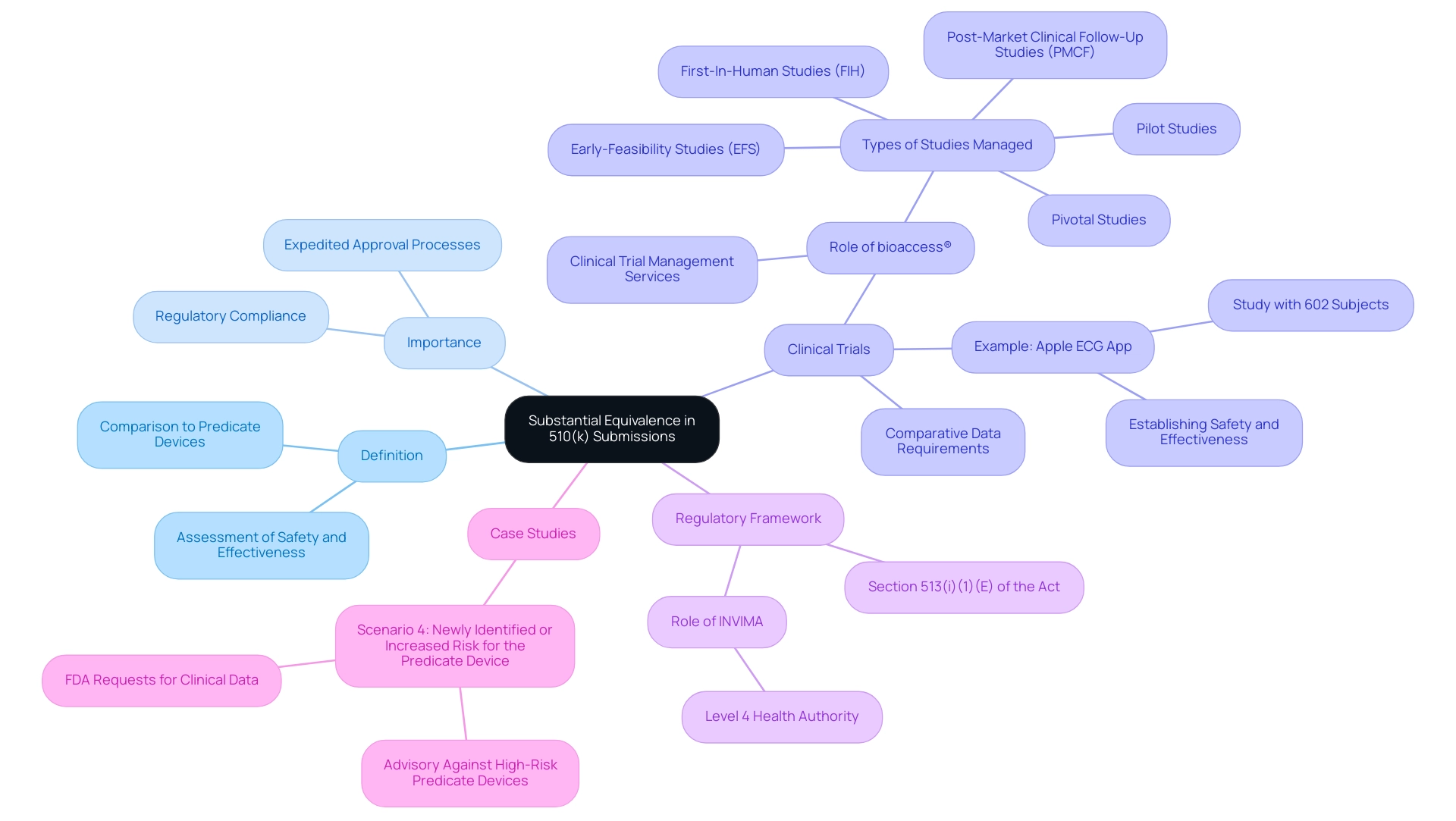
Substantial equivalence is defined as the assessment that a new health instrument is at least as safe and effective as a legally marketed predicate item. In the context of Latin America, leveraging comprehensive clinical trial management services, such as those offered by bioaccess®, is essential for manufacturers aiming to substantiate this claim. They are required to present comparative data that highlights similarities in design, materials, intended use, and performance characteristics.
This principle is crucial as it enables expedited approval processes for products that do not introduce new safety or effectiveness concerns. For instance, Apple conducted a clinical study with 602 subjects for its ECG App, which helped establish safety and effectiveness, underscoring the importance of robust comparative data. Additionally, Software as a Medical Device (SaMD) is increasingly relevant in this context, presenting unique considerations for demonstrating substantial equivalence.
By enabling quicker access to innovative medical technologies, substantial equivalence acts as a vital element in the oversight landscape. As noted by Tom Rish, 'Section 513(I)(1)(E) of the Act generally limits the determination of the intended use of an item that is the subject of a premarket notification (510(k)) to the proposed labeling contained in the submission.' This emphasizes the importance of aligning new equipment closely with their predicate counterparts to navigate the regulatory framework successfully.
Furthermore, understanding the role of INVIMA, the Colombia National Food and Drug Surveillance Institute, and its classification as a Level 4 health authority by PAHO/WHO is vital for compliance and oversight. The case study titled 'Scenario 4: Newly Identified or Increased Risk for the Predicate Product' illustrates the practical challenges manufacturers may face; it advises against selecting a predicate product with newly identified risks unless no alternatives are available. Considering that a considerable amount of predicate instruments are employed in what is 510k approval, it is crucial for manufacturers to comprehend how to efficiently illustrate substantial equivalence to simplify their submission methods.
bioaccess® specializes in managing various studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). With over 20 years of experience in Medtech, bioaccess® has the expertise and customized approach needed to navigate the complexities of clinical trials.
510(k) vs. Premarket Approval: Key Differences Explained
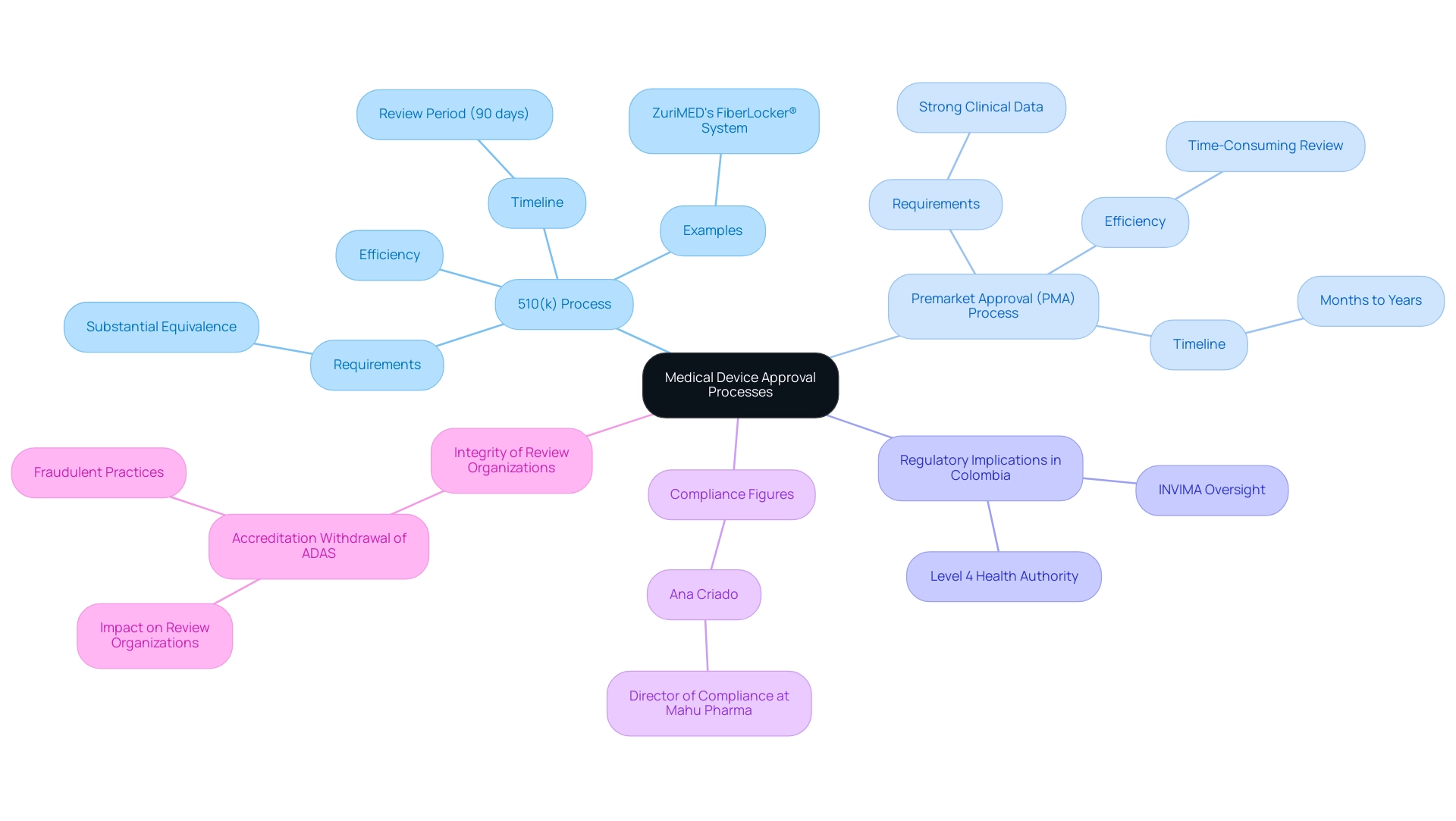
The 510(k) process is intended to be more efficient and less demanding than the Premarket Approval (PMA) process, which is mandatory for items classified as high-risk. A notable example of a successful 510(k) submission is ZuriMED's FiberLocker® system, which recently secured FDA clearance, demonstrating how the 510(k) pathway can facilitate quicker access to the market for certain products. The primary objective of 510(k) submissions, which relates to what is 510k approval, is to demonstrate substantial equivalence to already marketed products, thereby streamlining the approval pathway.
In contrast, PMA submissions require strong clinical data to validate the safety and efficacy of a new device, leading to a more extensive and time-consuming review. Typically, PMA submissions can take several months to years to complete, while 510(k) applications benefit from a comparatively swift review timeline, often within 90 days. As Sebastian Rodriguez-Elizalde, M.D., a member of the Scientific Advisory Board, observes, the differences in these procedures are essential for comprehending oversight pathways.
Moreover, the accreditation withdrawal of Accelerated Device Approval Services (ADAS) in August 2021 due to fraudulent practices underscores the significance of integrity among review organizations and its implications for the PMA system. This distinction emphasizes the pivotal role that risk classification plays in oversight pathways, influencing both the approval times and the complexity involved in the submission process. In Colombia, the regulatory framework is guided by INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), which oversees the market and manufacturing of health products, including health instruments.
The agency's classification as a Level 4 health authority by PAHO/WHO strengthens its ability to ensure the safety and efficacy of healthcare products. The Directorate for Healthcare Instruments and other Technologies within INVIMA plays a critical role in monitoring and controlling healthcare instruments, suggesting technical standards, and overseeing pre- and post-market programs. Ana Criado, a notable personality in compliance matters and the Director of Compliance at Mahu Pharma, offers invaluable knowledge in maneuvering through these intricate rules, especially in the realm of healthcare products and in vitro diagnostics.
Her extensive background in compliance practices positions her to contribute significantly to enhancing Colombia's oversight landscape, ensuring adherence and fostering innovation in healthcare technology.
Common Challenges in the 510(k) Submission Process and How to Overcome Them
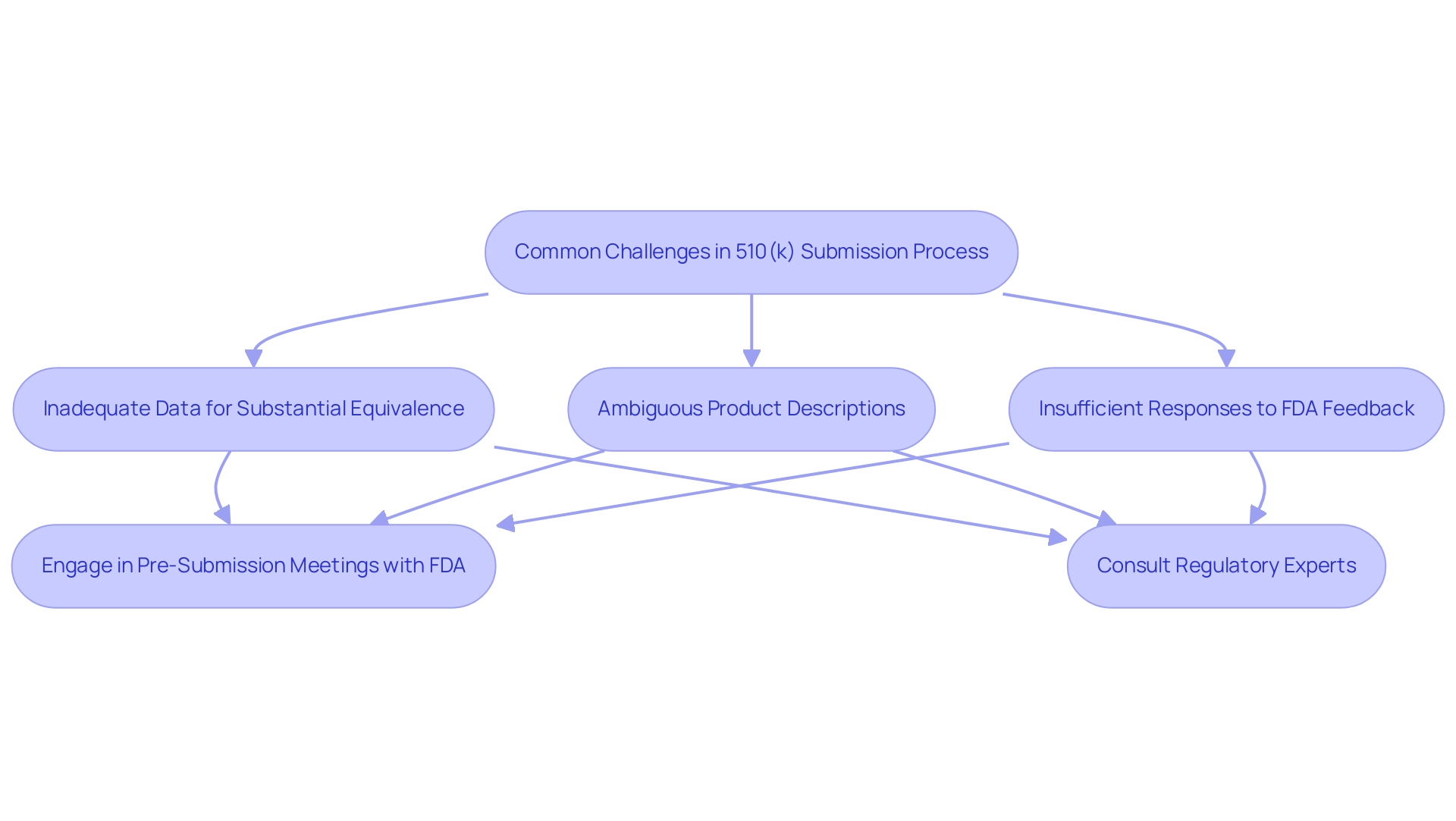
Understanding what 510k approval is essential, as the submission procedure is filled with challenges that can obstruct prompt approvals for medical products, especially for startups encountering regulatory obstacles, competition, and financial limitations. As noted, prior to the Refuse to Accept (RTA) policy, submissions to the FDA could lead to prolonged waiting periods—often months—before receiving feedback. Common hurdles include:
- Inadequate data to demonstrate substantial equivalence
- Ambiguous product descriptions
- Insufficient responses to FDA feedback
For instance, many submissions fail to adequately demonstrate equivalence to a predicate product, potentially necessitating a more complex de novo or Premarket Approval (PMA) process. A notable case study illustrates how the lack of sufficient equivalence data resulted in rejection, emphasizing the criticality of this requirement. Furthermore, new firms must register as an establishment with the FDA, incurring an annual fee, which contributes to the challenges encountered by newcomers in the healthcare equipment market.
Compounding these issues, medical device startups often struggle to secure clinical research sites, as many healthcare providers are hesitant to participate in trials involving new devices. Furthermore, they face stiff competition from established companies that possess greater brand recognition and existing relationships within the industry. To navigate these obstacles effectively, manufacturers are encouraged to engage in comprehensive pre-submission meetings with the FDA, which have been shown to positively impact approval rates.
These meetings facilitate a clearer understanding of regulatory expectations and provide tailored guidance. Regulatory consultants like Ana Criado offer essential services, including strategic advice on data collection and submission strategies, which can significantly enhance the likelihood of success. With her extensive experience and insights from her leadership roles and teaching positions, she provides critical expertise to address potential pitfalls proactively while helping startups navigate the competitive landscape.
Conclusion
The 510(k) approval process is a critical pathway for medical device manufacturers, facilitating the introduction of innovative products while ensuring patient safety. By demonstrating substantial equivalence to existing devices, this process streamlines market access for around 80% of Class II medical devices. A thorough understanding of the steps and requirements involved is essential for navigating the regulatory landscape effectively.
Substantial equivalence allows for expedited approvals of devices that do not introduce new safety concerns, emphasizing the need for robust comparative data in submissions. The differences between the 510(k) and Premarket Approval (PMA) processes further illustrate how risk classification influences regulatory pathways and timelines.
Manufacturers often encounter challenges, such as:
- Data inadequacies
- Communication issues with the FDA
Engaging regulatory experts and utilizing pre-submission meetings can significantly enhance the chances of successful outcomes. By proactively addressing these complexities, manufacturers can navigate the 510(k) process more effectively.
In summary, mastering the 510(k) approval process is not just a regulatory necessity; it is a strategic advantage that promotes innovation and improves patient outcomes in the medical device sector.
Frequently Asked Questions
What is the 510(k) authorization system?
The 510(k) authorization system, created by the U.S. Food and Drug Administration (FDA), allows medical equipment manufacturers to demonstrate that their products are 'substantially equivalent' to already legally marketed products, thus simplifying the path to market.
What is the main purpose of 510(k) approval?
The main purpose of 510(k) approval is to ensure that new medical products meet safety and efficacy standards without undergoing the more extensive Premarket Approval (PMA) process, which is typically for high-risk items.
Do all medical devices require a 510(k) submission?
No, low-risk Class I items may not require a 510(k) submission, while most Class II items do require it.
How significant is the Class II category in the medical device industry?
Approximately 80% of all medical devices fall under the Class II category, highlighting the critical role of the 510(k) approval process in the industry.
What services are important for navigating the 510(k) approval process?
Comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, are essential for navigating the compliance landscape effectively.
How long does it typically take to receive clearance for a 510(k) submission?
The average time to clearance for a 510(k) submission is approximately five to six months.
What is a Substantially Equivalent (SE) letter?
A Substantially Equivalent (SE) letter from the FDA permits the marketing of a product in the U.S., signifying that the product adheres to FDA regulations.
What are the essential steps involved in the 510(k) approval process?
The 510(k) approval process involves several essential steps, including confirming the need for a submission, compiling a comprehensive documentation package, and undergoing a thorough review by the FDA.
What recent trends have been observed in the approval times for medical devices?
Recent enhancements have shown a median approval time of 304 days for panel-track supplements in the first half of 2023, which is a 27% reduction compared to 2022 levels.
What factors can affect the length of the 510(k) approval process?
The length of the 510(k) approval process can vary significantly based on the complexity of the product and the completeness of the submission.

