Introduction
Navigating the regulatory landscape for medical devices can be a daunting task, particularly when it comes to the 510(k) submission process established by the FDA. This pathway is designed for devices that are deemed substantially equivalent to existing products, allowing manufacturers to gain market access while adhering to rigorous safety and efficacy standards.
A successful 510(k) submission requires meticulous preparation and a comprehensive understanding of the documentation process, which includes:
- Detailed information about the device's design
- Intended use
- Performance testing results
As the medical device industry continues to evolve, understanding the nuances of the 510(k) process is essential for manufacturers aiming to streamline their submissions and avoid common pitfalls that can lead to delays or denials.
This article delves into the intricacies of the 510(k) submission process, highlighting key types of submissions, the importance of substantial equivalence, and strategies to navigate potential challenges effectively.
Understanding the 510(k) Submission Process
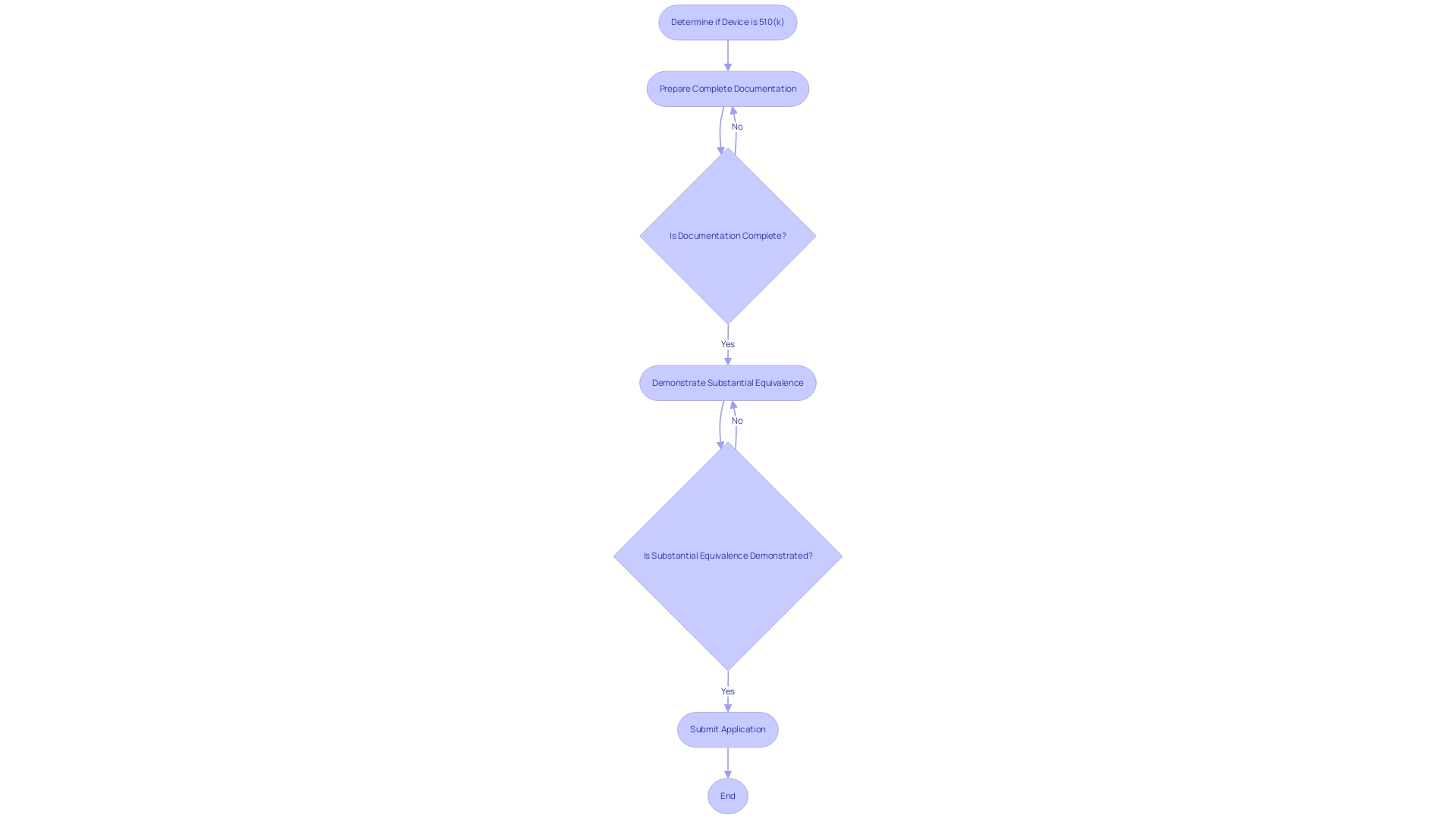
The process for determining what is a 510k medical device serves as a critical regulatory pathway established by the FDA for medical products that are considered substantially equivalent to existing legally marketed items. This pathway is vital for manufacturers seeking to obtain market access while adhering to stringent safety and efficacy standards. A complete 510(k) application must encompass detailed documentation, including the design, intended use, and results from performance testing.
Experts like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, emphasize the importance of substantiating that a product is as safe and effective as its predicate counterpart, a requirement essential for obtaining FDA clearance. Furthermore, Juan Cuya, MD, with his expertise in regulatory affairs and clinical trials, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, provide valuable insights into the complexities of the application process. The document detailing these regulations spans pages 48870 to 48878, providing a comprehensive reference for stakeholders.
Furthermore, ongoing efforts are necessary to enhance the 510(k) process to better mitigate potential risks to patients, underscoring the commitment to improving regulatory pathways in the medical device landscape. As noted by Olufunmilayo Ariyo from the FDA, 'For questions relating to this notice, please contact the Office of Financial Management.' Furthermore, it is important for applicants to note the standard premarket application fee of $483,560, which must be paid at the time of sending to avoid delays in the application process.
Understanding what is a 510k medical device involves recognizing common pitfalls in the 510(k) process, such as:
- Incomplete documentation
- Failure to demonstrate substantial equivalence
These issues can lead to delays or denials.
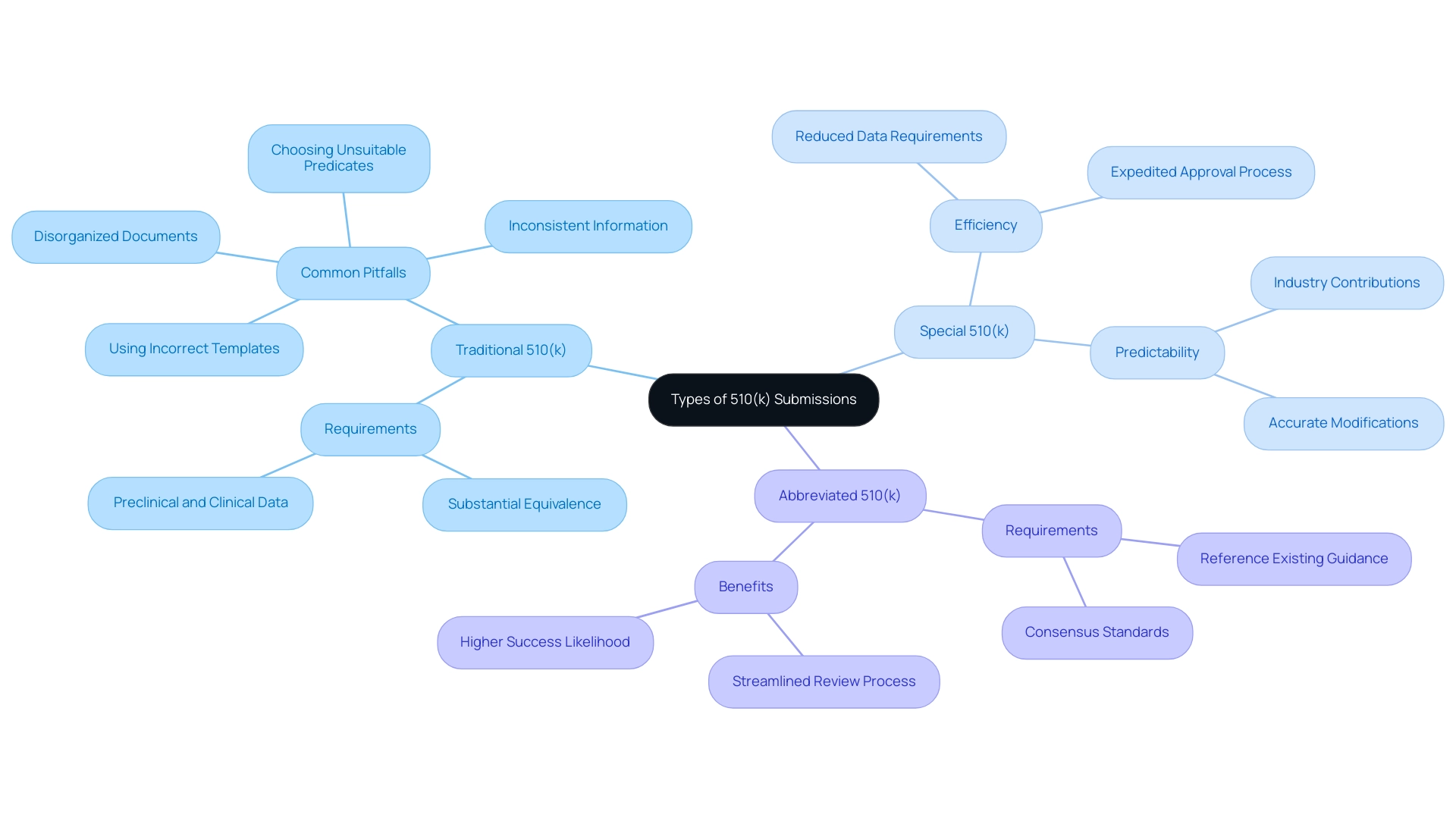
Types of 510(k) Submissions: Traditional, Special, and Abbreviated
-
Traditional 510(k): The standard 510(k) application is the most common route for medical equipment approval. It necessitates a thorough compilation of data to demonstrate substantial equivalence to a predicate device. This includes, when applicable, data derived from both preclinical and clinical studies to substantiate safety and effectiveness. In 2022, it was reported that 30% of 510(k) applications were not accepted for initial review, underscoring the critical need for meticulous preparation to avoid common pitfalls. These pitfalls often include:
- Using incorrect templates
- Choosing unsuitable predicates
- Inconsistent information
- Disorganized documents
These issues can significantly hinder the review process. With over 20 years of experience in Medtech, bioaccess® utilizes its expertise in managing clinical trials to help navigate these challenges effectively, ensuring documents are comprehensive and well-organized.
Special 510(k): This category is designed for products that have already received FDA approval and are undergoing minor modifications. The special 510(k) process is designed to facilitate a more efficient review by requiring a reduced amount of data, thereby expediting the approval process while still safeguarding the device’s safety and efficacy. Bradley Merrill Thompson emphasizes the predictability of industry contributions by stating,
Big picture, the first thing that strikes me in these box plots is that industry is much more predictable than FDA.
This insight highlights the significance of grasping the nuances of the application process, as predictability can lead to better preparation and outcomes. Engaging with specialized services for clinical trial management, like those offered by bioaccess®, can enhance this predictability and ensure that modifications are accurately reflected in submissions.
-
Abbreviated 510(k): The abbreviated 510(k) pathway is available for products that can reference existing FDA guidance documents or recognized consensus standards. This approach requires less data, aiming to streamline the review process while ensuring compliance with FDA regulations. By utilizing established standards, manufacturers can not only expedite their process but also enhance the likelihood of a successful outcome. As the landscape of 510(k) applications evolves, staying informed about updates and the differences between traditional, special, and abbreviated pathways is essential for clinical research directors navigating product approvals. Collaboration with experienced trial management teams, such as those at bioaccess®, can further ensure that submissions are well-prepared, addressing common mistakes and facilitating faster market access.
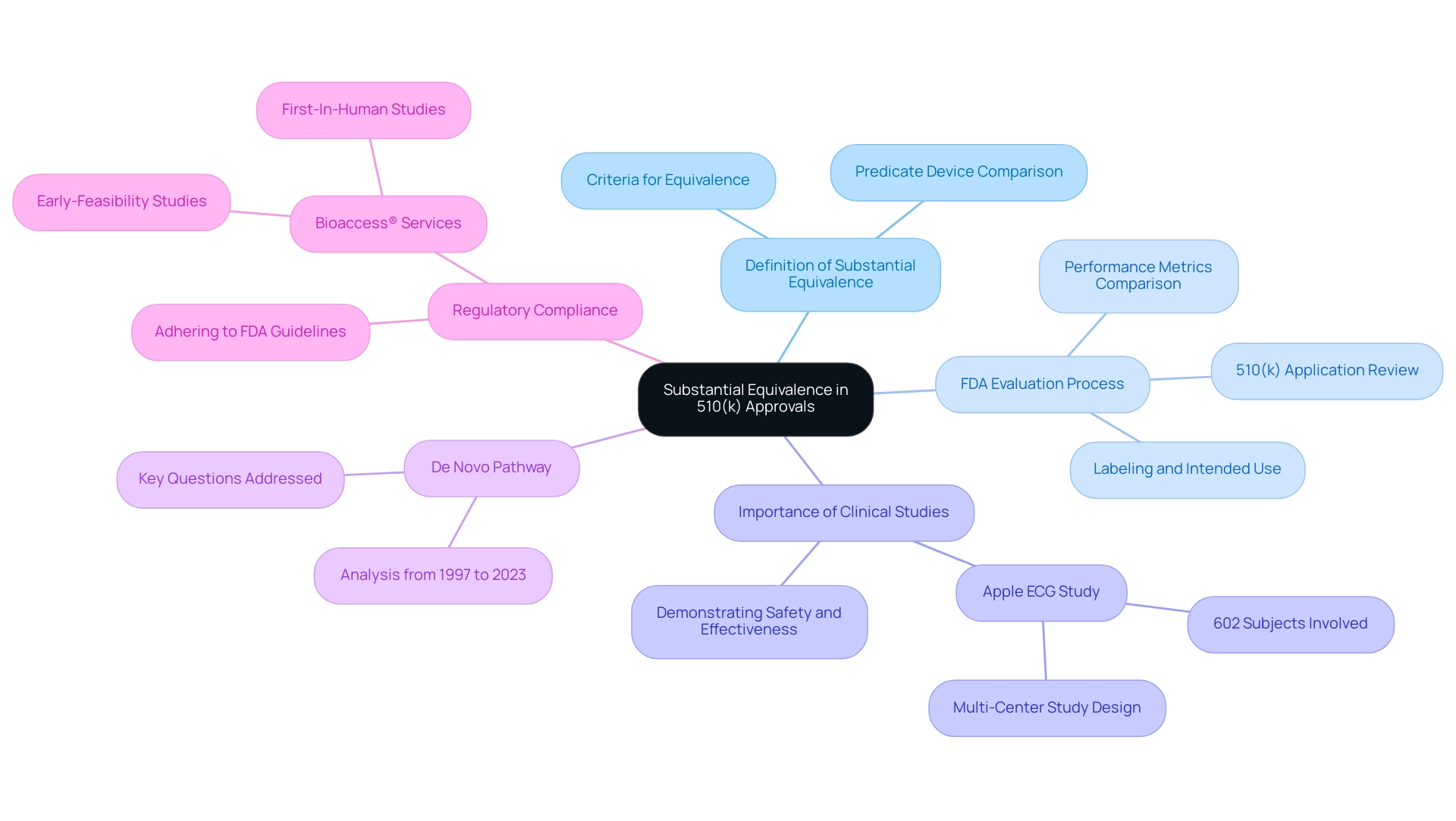
The Role of Substantial Equivalence in 510(k) Approvals
Substantial equivalence is defined as a determination that a new medical instrument is as safe and effective as a legally marketed predicate instrument. To establish this equivalence, manufacturers must provide comprehensive evidence demonstrating that their product shares the same intended use and technological characteristics as the predicate. Alternatively, if there are differences, these must not introduce new questions regarding safety or effectiveness.
The FDA's evaluation process involves a meticulous review of the information presented within the 510(k) application, which helps to clarify what is a 510k medical device through comparisons of performance metrics, labeling details, and intended use scenarios. A notable case is Apple's clinical study for the ECG App, which involved a prospective, multi-center pivotal study with 602 subjects across five investigative sites. Specifically, they conducted a prospective, parallel-cohort, multi-center pivotal study using an enriched population of 602 subjects at 5 investigational sites.
This research was crucial in demonstrating the safety and effectiveness of the apparatus, highlighting what is a 510k medical device's essential importance of substantial equivalence in regulatory filings. Furthermore, understanding the analysis of the De Novo pathway from 1997 to 2023 is essential, as it addresses ten key questions about its application, thereby providing context for substantial equivalence. Adhering to FDA regulations and guidance documents is essential for medical product manufacturers to align their applications with FDA expectations, enhancing the chances of successful approvals.
By leveraging the comprehensive clinical trial management services offered by bioaccess®, including expertise in Early-Feasibility Studies and First-In-Human Studies led by professionals like Katherine Ruiz, applicants can enhance their compliance with FDA regulations. This expertise not only clarifies the complexities of the application process but also emphasizes the importance of substantial equivalence, forming the foundation of the FDA's decision-making process regarding device approvals. Clients can anticipate enhanced results and streamlined procedures, ultimately increasing their chances of successful FDA approvals.
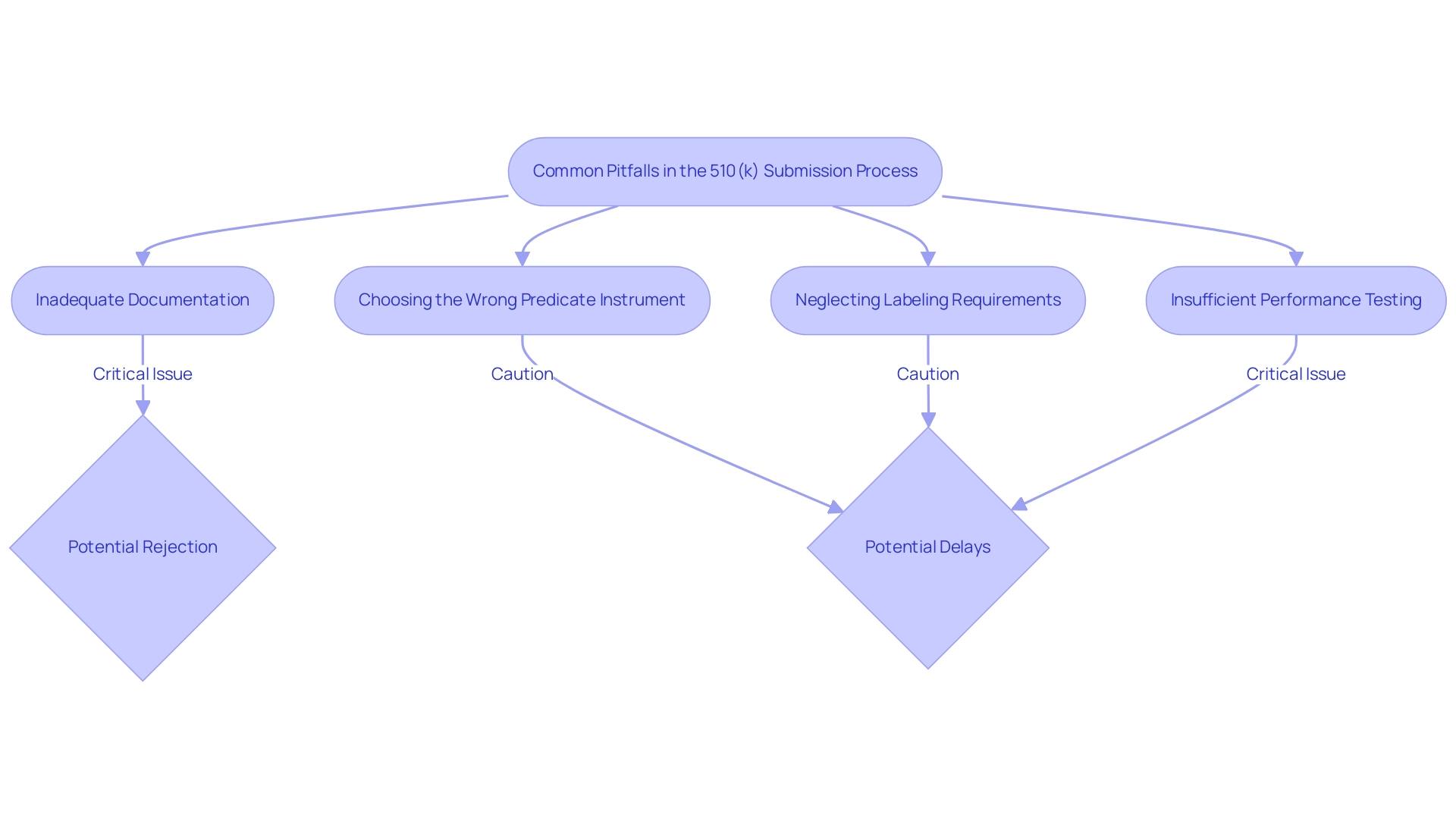
Common Pitfalls in the 510(k) Submission Process
- One of the leading causes of 510(k) application rejections is inadequate documentation related to what is a 510k medical device. Submitting incomplete data or failing to adhere to the FDA's established guidelines can significantly jeopardize the approval process. In fact, it is estimated that preparing a comprehensive document can take around 100 hours, underscoring the need for meticulous attention to detail. Ensuring that all required documentation is thorough and meets FDA standards is crucial for a successful application. As noted, prior to the Refuse to Accept (RTA) process, you would submit your 510(k) to the FDA, which raises the question of what is a 510k medical device, and often wait months before receiving any feedback, highlighting the importance of timely and complete entries. Cases have shown that entries lacking necessary information often face rejection, emphasizing the critical importance of adequate documentation. Our comprehensive clinical trial management services, led by experts like Katherine Ruiz, specialize in providing feasibility studies, site selection, compliance reviews, and project management to ensure that documentation is not only complete but also adheres to all regulatory requirements. We also focus on the reporting of study status, inventory, and serious and non-serious adverse events, ensuring a holistic approach to trial management.
- Choosing the Wrong Predicate Instrument: Selecting an unsuitable predicate instrument can severely affect the success of what is a 510k medical device application. It is essential to conduct thorough research to ensure that the chosen predicate is comparable in terms of intended use, design, and technological characteristics. Failure to properly identify a suitable predicate may lead to unnecessary delays and complications in the approval process.
- Neglecting Labeling Requirements: A crucial aspect of understanding what is a 510k medical device is that labeling is a vital component of the 510(k) submission. Inaccuracies or omissions in labeling can result in compliance issues and may even lead to rejection. It is important to ensure that labeling meets FDA requirements and accurately reflects the product’s intended use. Clear and precise labeling not only aids in regulatory compliance but also enhances user understanding, further supporting the product's safety and effectiveness claims.
Insufficient Performance Testing: Providing insufficient performance data can lead to significant delays in the review process. The FDA mandates thorough performance testing to showcase the safety and efficacy of a product. It is crucial to conduct the necessary tests and include detailed results as part of the presentation. Failing to adequately demonstrate performance can result in requests for additional information, prolonging the time it takes for a device to reach the market. According to the case study titled 'Common 510(k) Submission Mistakes,' a significant percentage of entries face rejection due to common errors related to what is a 510k medical device, such as inadequate testing documentation, emphasizing the need for thorough performance evaluations. Leveraging our clinical trial management expertise can help navigate these challenges effectively, from trial setup to ongoing monitoring and reporting on all critical aspects of the study.
Navigating the FDA Review Timeline for 510(k) Submissions
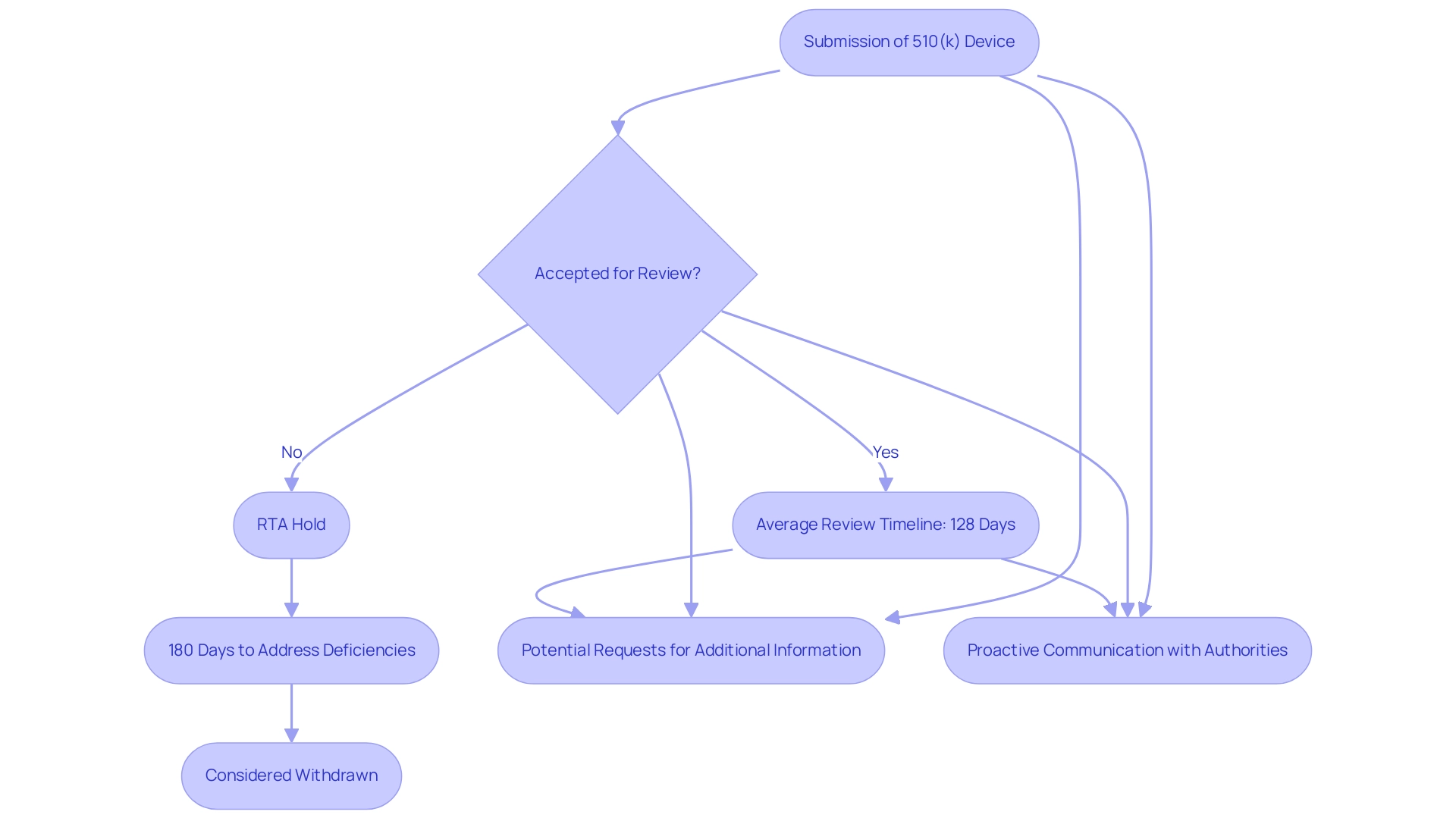
The FDA aims to make decisions on applications regarding what is a 510k medical device within an average of 128 calendar days during its 2023 financial year. However, this timeline can vary based on several factors, including the intricacy of the apparatus and the thoroughness of the proposal. Following the submission of what is a 510k medical device, if it is not accepted for review, it is placed on RTA Hold, and manufacturers have 180 days to address any deficiencies before it is considered withdrawn.
It is essential for manufacturers to understand that regulatory processes in Colombia, overseen by INVIMA (Colombia National Food and Drug Surveillance Institute), align with those in the U.S. INVIMA, designated as a Level 4 health authority by PAHO/WHO, supervises the marketing and production of health products, including medical items, ensuring adherence to safety and efficacy standards. Within INVIMA, the Directorate for Medical Devices and other Technologies plays a crucial role in monitoring and controlling medical devices, suggesting technical standards, and overseeing pre- and post-market programs. Manufacturers should also be prepared for potential requests for additional information or clarification from both the FDA and INVIMA, which can further extend the review period.
It is crucial for applicants to anticipate these delays and maintain proactive communication with regulatory authorities to ensure a more streamlined review process. For instance, ZuriMED Technologies AG recently secured FDA clearance for its Fiber Locker® System, illustrating the successful navigation of the review process. As emphasized by industry expert Nick Paul Taylor, companies must be vigilant in ensuring compliance with quality systems to avoid setbacks during the review.
By thoroughly planning and understanding the intricacies of the review timelines in both jurisdictions, manufacturers can significantly enhance the likelihood of a successful submission. Additionally, Regulatory Affairs experts like Ana Criado, who has extensive experience with INVIMA and serves as a consultant for major global firms, can provide valuable insights into navigating these complex regulatory landscapes.
Conclusion
Navigating the 510(k) submission process is an essential endeavor for medical device manufacturers aiming to achieve FDA clearance efficiently. This article has elucidated the critical components of the 510(k) pathway, emphasizing the importance of meticulous documentation, the necessity of demonstrating substantial equivalence, and the various types of submissions, including traditional, special, and abbreviated 510(k) applications. Each submission type has its own nuances that can significantly impact the approval timeline and success rate, underscoring the need for thorough preparation.
The significance of substantial equivalence cannot be overstated, as it forms the cornerstone of the FDA's evaluation process. Manufacturers must provide robust evidence that their devices are as safe and effective as existing predicate devices. Common pitfalls, such as inadequate documentation, improper predicate selection, and insufficient performance testing, can lead to delays or rejections. Recognizing and addressing these challenges proactively is vital for a successful submission.
Ultimately, understanding the intricacies of the 510(k) process and maintaining compliance with FDA regulations is crucial for manufacturers. Leveraging expert guidance and comprehensive clinical trial management services can enhance submission quality and expedite market access. By adopting a strategic approach to the 510(k) submission process, manufacturers can navigate regulatory requirements effectively, ensuring their devices reach the market while upholding the highest standards of safety and efficacy.
Frequently Asked Questions
What is a 510(k) medical device?
A 510(k) medical device is a product that the FDA considers substantially equivalent to existing legally marketed items, allowing manufacturers to obtain market access while adhering to safety and efficacy standards.
What is required for a complete 510(k) application?
A complete 510(k) application must include detailed documentation such as the device design, intended use, and results from performance testing.
What are some common pitfalls in the 510(k) process?
Common pitfalls include incomplete documentation, failure to demonstrate substantial equivalence, using incorrect templates, choosing unsuitable predicates, inconsistent information, and disorganized documents.
What are the three types of 510(k) submissions?
The three types of 510(k) submissions are Traditional 510(k), Special 510(k), and Abbreviated 510(k).
What is the Traditional 510(k) submission?
The Traditional 510(k) is the most common route for medical equipment approval, requiring comprehensive data to demonstrate substantial equivalence to a predicate device.
What is a Special 510(k)?
A Special 510(k) is for products that have already received FDA approval and are undergoing minor modifications, requiring less data for a more efficient review process.
What is an Abbreviated 510(k)?
The Abbreviated 510(k) pathway is for products that can reference existing FDA guidance documents or recognized consensus standards, requiring less data to streamline the review process.
What is the standard premarket application fee for a 510(k)?
The standard premarket application fee for a 510(k) is $483,560, which must be paid at the time of submission to avoid delays.
Who can provide insights into the 510(k) application process?
Experts like Ana Criado, Juan Cuya, and Katherine Ruiz provide valuable insights into the complexities of the 510(k) application process.
What ongoing efforts are being made regarding the 510(k) process?
Ongoing efforts aim to enhance the 510(k) process to better mitigate potential risks to patients and improve regulatory pathways in the medical device landscape.

