Introduction
In the realm of medical devices and treatments, clinical evaluation reports (CERs) stand as a cornerstone of regulatory compliance and patient safety. These detailed documents meticulously assess the safety and effectiveness of medical innovations, serving not only as a testament to a product's reliability but also as a vital communication tool among stakeholders, including healthcare providers and regulatory authorities.
Particularly in Latin America, where unique challenges abound, the significance of CERs is amplified as they navigate complex regulatory landscapes. With the increasing reliance on evidence-based practices, the integration of comprehensive CERs is becoming essential for Medtech companies aiming to secure approvals and ensure ongoing patient safety.
This article delves into the critical components, regulatory frameworks, and practical applications of CERs, illuminating their vital role in advancing healthcare standards and fostering informed decision-making in clinical settings.
Defining Clinical Evaluation Reports: An Overview
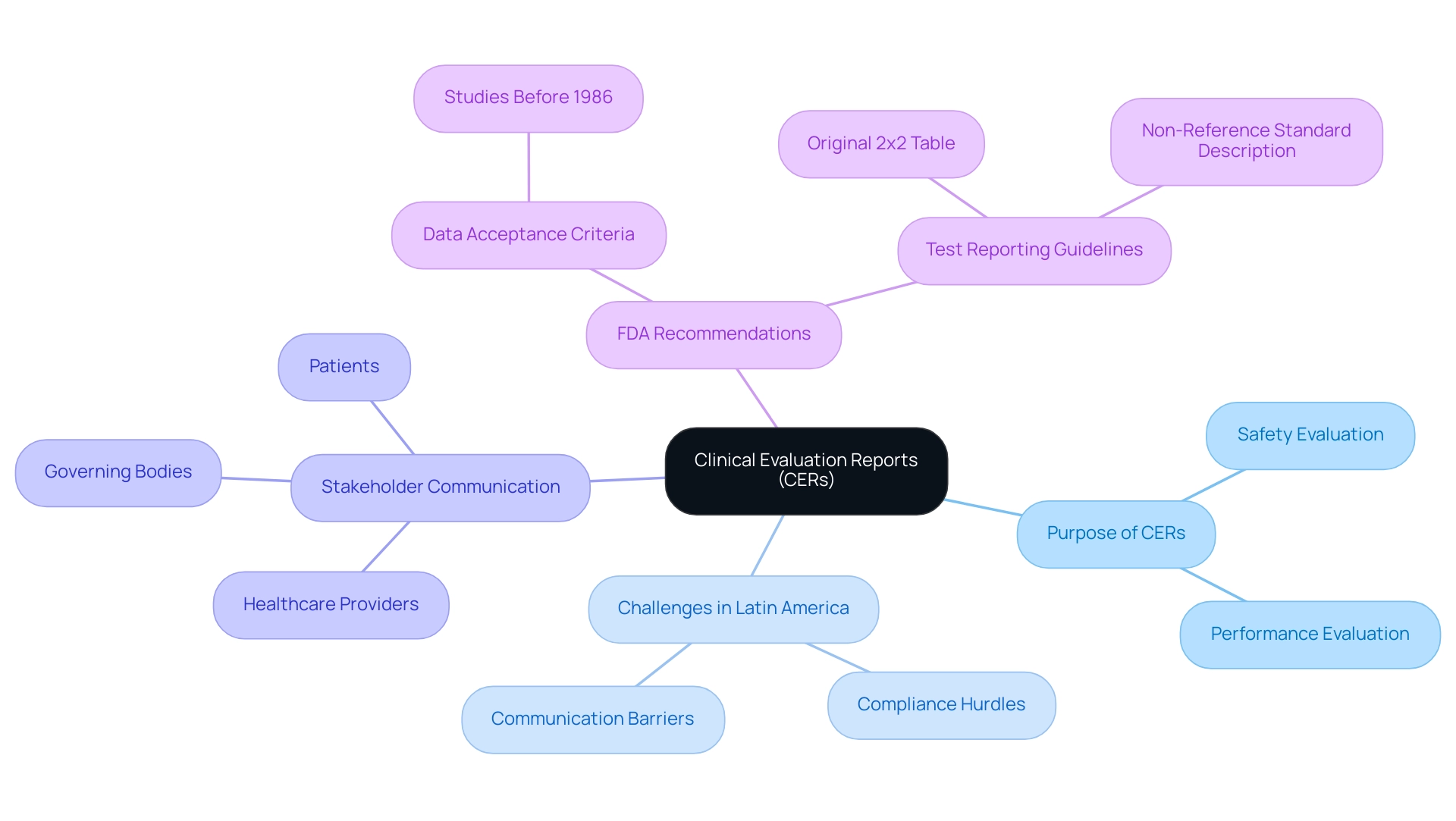
Clinical evaluation reports (CERs) are essential documents that meticulously evaluate the safety and performance of medical devices or treatments by utilizing a clinical evaluation format based on clinical data. These reports play a crucial role in the approval process, particularly in the context of Latin America, where Medtech companies encounter unique challenges such as compliance hurdles and communication barriers. CERs not only act as proof of a product's effectiveness but also aid in conveying essential information to various stakeholders, including healthcare providers, governing bodies, and patients.
For instance, Maria Nyåkern, Ph.D., has managed dozens of research investigations involving tens of thousands of patients, underscoring the real-world impact of Cars on patient care and product validation. With the expertise of firms like bioaccess®, which specializes in Early-Feasibility, First-In-Human, and Post-Market Follow-Up Studies, the integration of comprehensive CERs can significantly bolster ongoing post-market surveillance efforts. Moreover, recent statistics indicate a growing prevalence of CERs in medical device approvals, reflecting an industry shift towards rigorous evidence-based practices.
The FDA acknowledges the legitimacy of data from studies initiated prior to November 19, 1986, as long as it represents valid scientific evidence and does not infringe upon the rights, well-being, and welfare of human subjects, demonstrating adaptability in the assessment process. GRADE, a 'level of evidence' according to Cochrane, emphasizes the importance of quantifying the scientific quality of publications related to these reports. Moreover, the FDA's suggestions on test reporting highlight the importance of accurately presenting original test results to prevent misinterpretation, thus improving the trustworthiness of medical assessments.
The FDA advises against revising original test results based on unverified assumptions, instead recommending the reporting of the original 2x2 table of results along with a description of the non-reference standard and appropriate agreement measures. Ultimately, the integration of comprehensive CERs into the clinical evaluation format not only aids in gaining necessary approval but also significantly influences patient safety statistics, highlighting their vital importance in the medical device field, especially in navigating the complexities of research studies in Latin America.
Key Components and Structure of Clinical Evaluation Reports
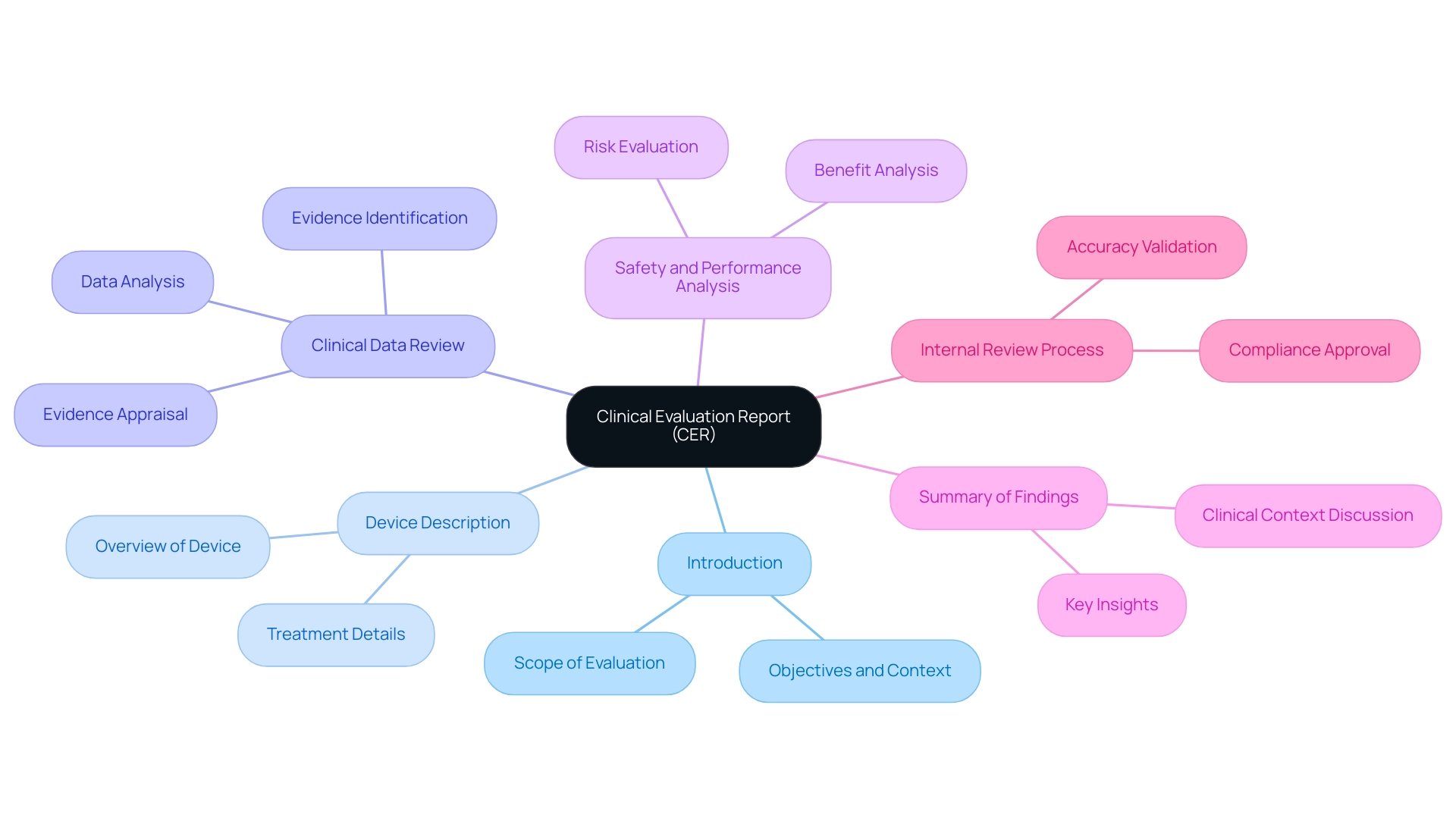
A well-organized clinical evaluation format is essential for the successful appraisal and sale of medical devices in the European Union, as it records the medical assessment necessary for regulatory approval. The CER typically encompasses six critical components. Firstly, the introduction delineates the scope of the evaluation, clearly defining the objectives and context of the assessment.
Following this, a comprehensive description of the medical device or treatment under review is provided, which sets the foundation for the subsequent analysis. The heart of the report contains a detailed review of the clinical data, where clinical evidence is identified, appraised, and analyzed in accordance with the protocols specified in the clinical evaluation format. This is essential for ensuring the reliability and performance of the device.
An analysis of the evidence regarding safety and performance is imperative, allowing stakeholders to understand the risks and benefits associated with the device. The report concludes with a summary of findings, encapsulating the key insights gleaned from the evaluation. Furthermore, it might encompass a discussion of the clinical context, risk evaluation, and citations to pertinent literature, all within the clinical evaluation format, which improves the report's depth and adherence to standards.
Significantly, the finalized CER must go through an internal review process to ensure accuracy and compliance, with necessary approvals obtained from oversight authorities before submission. This internal review is essential for validating the report's findings and ensuring compliance with official requirements. Each component must be meticulously crafted to ensure not only the informative nature of the report but also adherence to the necessary compliance requirements, thereby facilitating a smoother review and approval process.
Utilizing extensive clinical trial management services, such as:
- Feasibility studies
- Site selection
- Compliance assessments
- Trial preparation
- Import permits
together with the knowledge of experts like Katherine Ruiz, improves the effectiveness of the clinical assessment process, establishing bioaccess® as a top Medtech clinical research organization in Latin America dedicated to innovation and compliance excellence.
The Purpose of Clinical Evaluations in Healthcare
Clinical assessments play an essential role in healthcare, helping to determine the reliability and efficacy of medical devices and treatments. These assessments produce crucial proof that backs regulatory filings, guaranteeing that products fulfill strict health standards before patient exposure. They enrich the existing body of medical knowledge, enabling healthcare professionals to make well-informed decisions regarding treatment options.
By systematically reviewing medical data, including the calculation of confidence intervals around various statistics, evaluations enhance the reliability of findings, identifying potential risks and benefits associated with interventions. This rigorous approach ultimately contributes to enhanced patient safety and care quality.
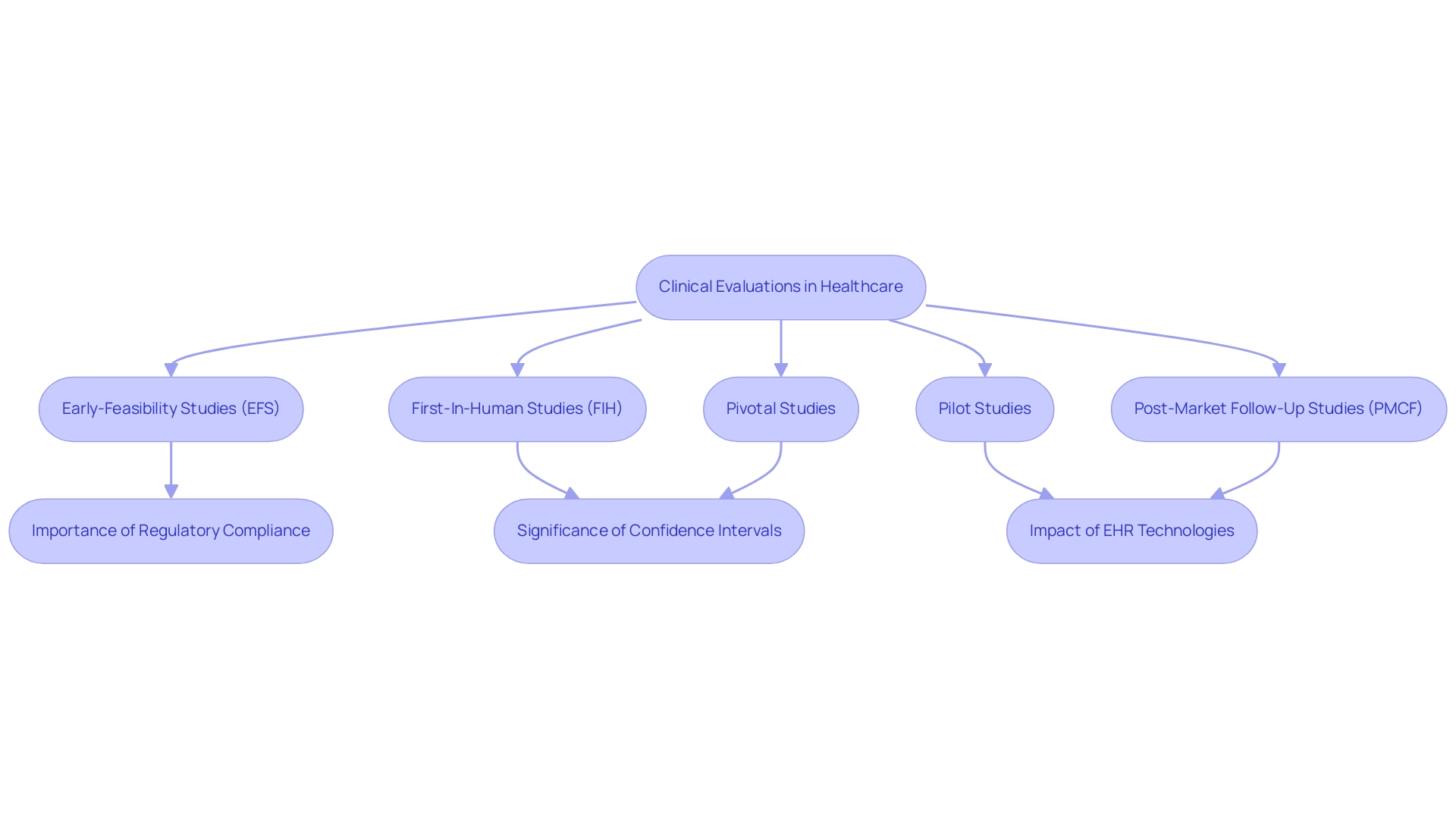
In Latin America, companies like bioaccess® leverage over 20 years of expertise in managing trials with a flexible and customized approach, focusing on a range of studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
Their comprehensive trial management services encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, ensuring that all regulatory requirements are met.
The application of confidence intervals is particularly significant, as they provide a measure of the stability of the point estimates derived from medical data, guiding practitioners in their decision-making processes. A significant illustration is the beta-blocker assessment of bucindolol in individuals with advanced chronic heart failure, published in the New England Journal of Medicine in 2001, demonstrating the importance of medical assessments in determining treatment efficacy. Recent practices in post-trial analysis further emphasize the need for long-term follow-up, monitoring, and post-marketing surveillance, which are vital in assessing treatment durability and profiles.
Furthermore, a recent study sampling 21 health systems and 32 ambulatory care practices highlighted the impact of diverse electronic health record technologies on the generalizability of findings. This highlights the importance of medical assessments in improving patient safety across various healthcare settings, as the differences in technologies employed can affect the results and relevance of the findings.
Regulatory Framework for Clinical Evaluations
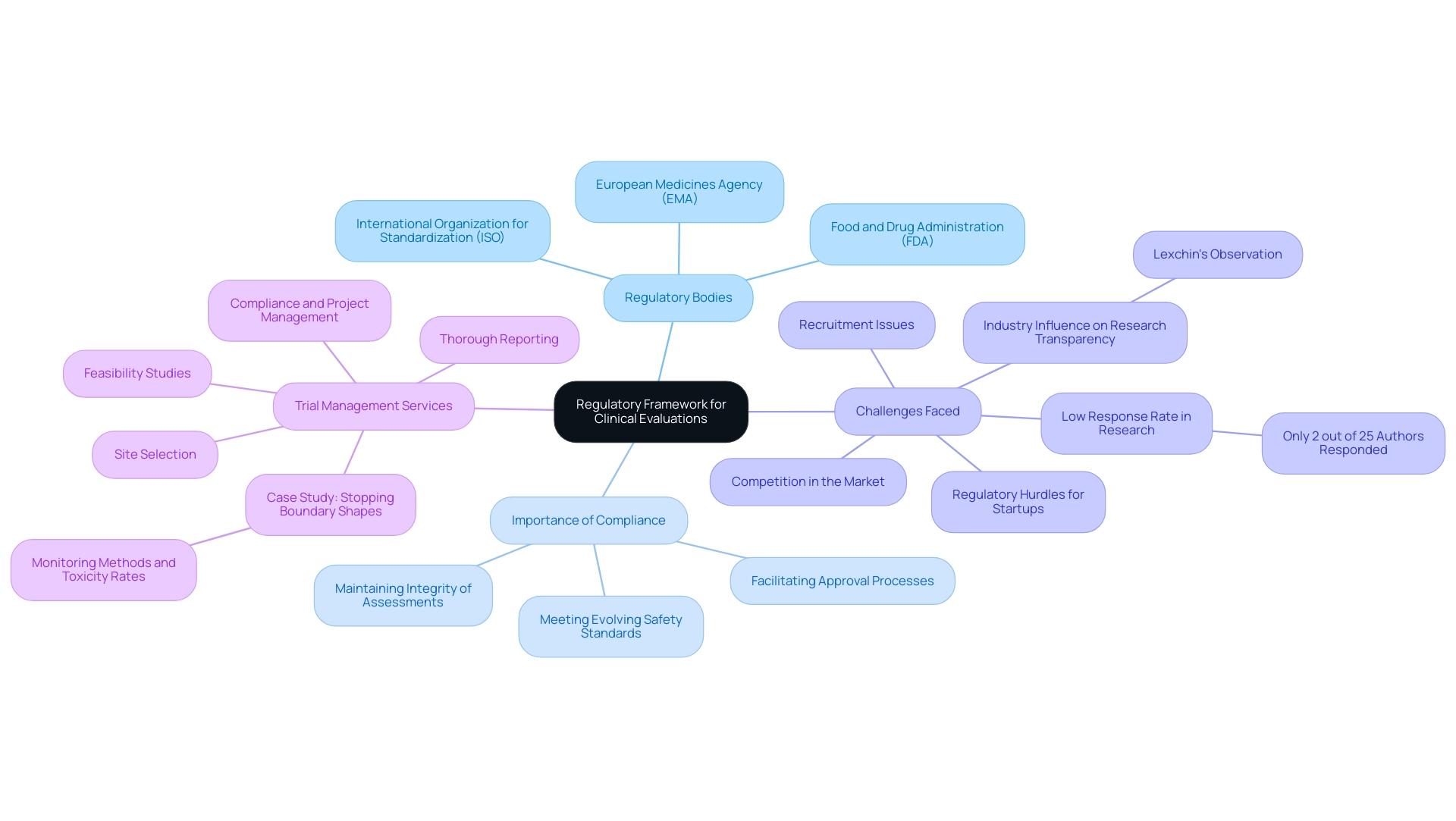
The regulatory framework overseeing medical assessments is influenced by several essential guidelines and standards, notably those set by the International Organization for Standardization (ISO) and the European Medicines Agency (EMA). In the United States, the Food and Drug Administration (FDA) plays a crucial role in establishing the criteria for medical assessments. These regulations outline the methodologies to be employed in assessments, specify the types of data that must be incorporated, and dictate the clinical evaluation format for the reports produced.
Adherence to these governing frameworks is not just a procedural formality; it is essential to maintaining the integrity of the assessment process and obtaining the necessary approvals from oversight authorities. Recent discussions among experts have emphasized that as of 2024, following current guidelines is essential to address evolving safety and efficacy standards. This adherence guarantees that medical assessments meet the stringent expectations established by both ISO and EMA, thus facilitating successful outcomes in the approval processes.
However, challenges persist, particularly for medical device startups navigating regulatory hurdles, competition, and recruitment issues. An examination of therapies for diabetic neuropathy highlighted the challenges in gathering thorough data for medical assessments, where only 2 out of 25 authors replied to inquiries for additional information. Moreover, Lexchin's observation that faculty members with industry backing were nearly twice as likely to decline requests for sharing research results highlights the impact of industry connections on research transparency, which is essential for evaluations in the medical field.
Furthermore, our extensive trial management services, directed by specialists such as Ana Criado, Director of Compliance Affairs, and Katherine Ruiz, a specialist in Compliance Affairs for Medical Devices, seek to address these challenges through feasibility studies, site selection, compliance, project management, and thorough reporting. Our services include thorough review and feedback on study documents to ensure compliance with country requirements, assistance with import permits and nationalization of investigational devices, and comprehensive reporting on study status and both serious and non-serious adverse events. The case study 'Stopping Boundary Shapes' illustrates the practical applications of varying monitoring methods under expected toxicity rates, demonstrating how different approaches can be tailored to meet specific trial needs while ensuring adherence to standards.
Practical Applications of Clinical Evaluation Reports
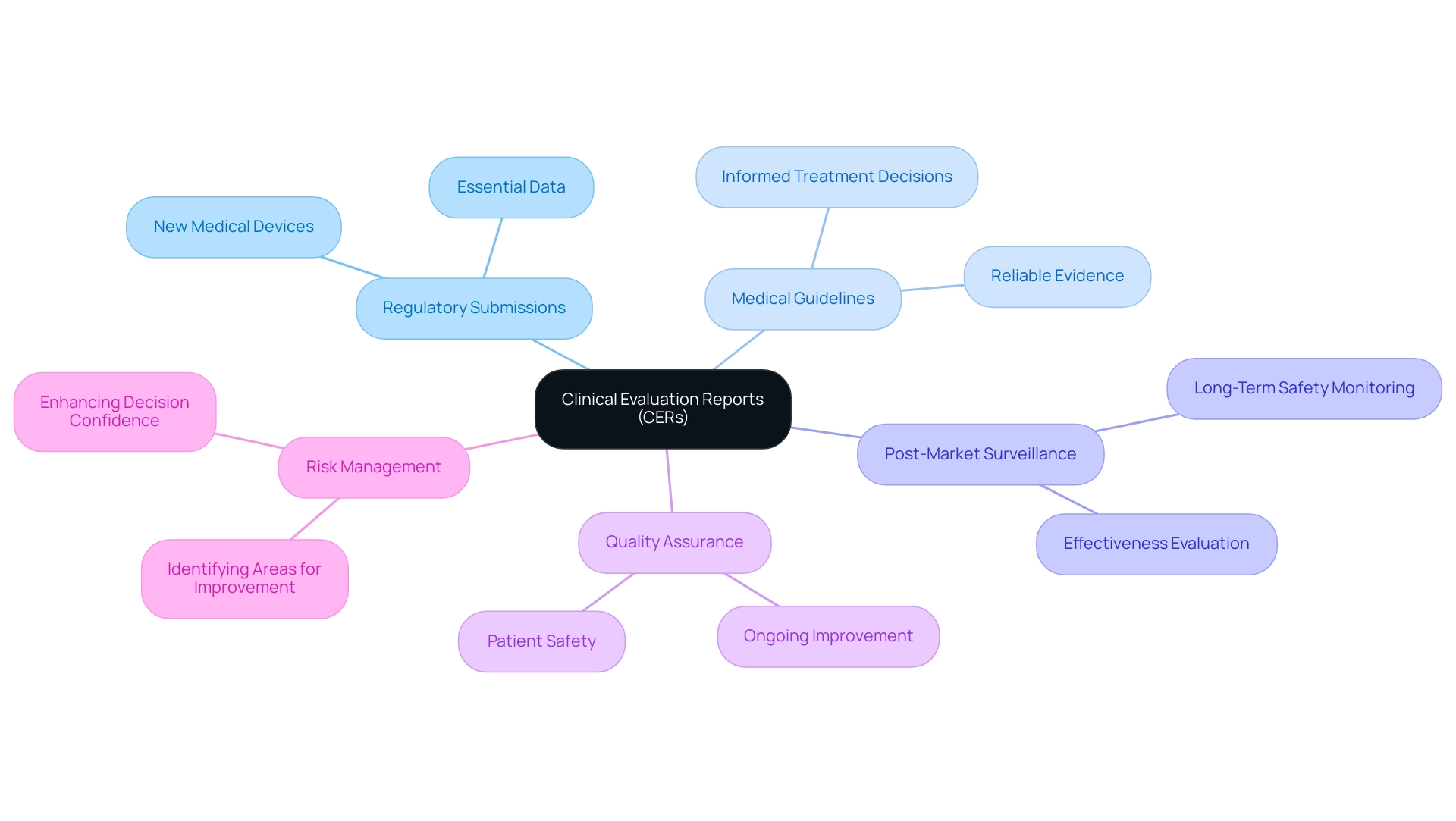
Evaluation reports (CERs) serve a critical role across numerous practical applications within the healthcare landscape, particularly in the context of accelerated medical device studies in Latin America, according to the clinical evaluation format. Primarily, they are crucial to regulatory submissions for new medical devices, as they provide essential data in a clinical evaluation format that regulators depend on to evaluate effectiveness and risk. In addition to this, the clinical evaluation format of CERs informs medical guidelines, ensuring that healthcare professionals have access to reliable evidence when considering new treatments.
The latest applications of CERs extend to supporting robust post-market surveillance activities, which are crucial in the clinical evaluation format for monitoring the long-term safety and effectiveness of medical devices once they are in use. For example, the knowledge of bioaccess®, supported by over 20 years of experience in Medtech, in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Post-Market Follow-Up Studies (PMCF), emphasizes the significance of an appropriate clinical evaluation format for the evaluation of evidence in making informed healthcare decisions. Moreover, healthcare professionals rely on the clinical evaluation format to assess the validity of new treatments and devices, ensuring that decisions are based on solid medical evidence.
In this way, CERs are foundational elements in ongoing quality assurance and risk management processes, which utilize a clinical evaluation format to enable the identification of areas for improvement within medical practice and reinforce patient safety as a paramount concern. Additionally, bioaccess® employs a customized clinical evaluation format to navigate trials effectively, enhancing the likelihood of successful outcomes. As disease prevalence affects predictive values, understanding these dynamics through CERs enhances confidence in clinical decisions made using a clinical evaluation format.
For instance, the relationship between disease prevalence and predictive values is crucial, as illustrated in the clinical evaluation format of the case study on Positive and Negative Predictive Values, which emphasizes how increasing disease prevalence raises positive predictive value (PPV) while decreasing negative predictive value (NPV). This illustrates the tangible impact of these reports within the clinical evaluation format. For reference, the PMCID for the article is PMC4900305 and the PMID is 25566715.
Conclusion
The importance of clinical evaluation reports (CERs) in the medical device landscape cannot be overstated. These comprehensive documents serve as a foundation for regulatory compliance, ensuring that medical innovations are both safe and effective before they reach patients. The rigorous structure of CERs, which includes critical components such as:
- Clinical data analysis
- Risk assessment
- Compliance verification
is essential for navigating the complex regulatory environments, particularly in Latin America.
Moreover, CERs play a pivotal role in fostering informed decision-making among healthcare professionals by providing reliable evidence that informs clinical guidelines and practices. As the demand for transparency and accountability in healthcare grows, the integration of robust CERs into the approval process not only enhances patient safety but also aligns with the increasing emphasis on evidence-based practices in the medical field.
In conclusion, the integration of comprehensive clinical evaluation reports is vital for Medtech companies striving to meet regulatory standards and enhance patient safety. By ensuring that all aspects of medical device evaluation are meticulously documented and assessed, stakeholders can navigate the regulatory landscape more effectively, ultimately leading to improved healthcare outcomes. As the industry continues to evolve, the significance of CERs will only increase, further solidifying their role as a cornerstone in the advancement of medical technology and patient care.
Frequently Asked Questions
What are Clinical Evaluation Reports (CERs)?
Clinical Evaluation Reports (CERs) are essential documents that evaluate the safety and performance of medical devices or treatments using clinical data. They play a crucial role in the approval process, particularly in Latin America, where they help address compliance challenges and communication barriers.
Why are CERs important in the medical device approval process?
CERs serve as proof of a product's effectiveness and convey essential information to stakeholders, including healthcare providers, governing bodies, and patients. They are increasingly prevalent in medical device approvals, reflecting a shift towards rigorous evidence-based practices.
What are the critical components of a Clinical Evaluation Report?
A CER typically includes six critical components: an introduction outlining the evaluation's scope, a description of the medical device or treatment, a detailed review of clinical data, an analysis of safety and performance evidence, a summary of findings, and potentially a discussion of clinical context and risk evaluation.
How does the FDA view data from studies initiated before November 19, 1986?
The FDA acknowledges the legitimacy of data from studies initiated prior to this date as long as it represents valid scientific evidence and does not infringe on the rights and welfare of human subjects.
What is the significance of the GRADE system in relation to CERs?
GRADE, a 'level of evidence' system, emphasizes the importance of quantifying the scientific quality of publications related to CERs, enhancing the credibility of the evidence presented.
What recommendations does the FDA provide regarding test reporting in CERs?
The FDA advises against revising original test results based on unverified assumptions and recommends reporting the original 2x2 table of results along with a description of the non-reference standard and appropriate agreement measures to improve the trustworthiness of medical assessments.
What is the internal review process for a finalized CER?
The finalized CER must undergo an internal review process to ensure accuracy and compliance, with necessary approvals obtained from oversight authorities before submission, validating the report's findings and adherence to official requirements.
How can clinical trial management services enhance the CER process?
Utilizing extensive clinical trial management services, such as feasibility studies, site selection, compliance assessments, and trial preparation, improves the effectiveness of the clinical assessment process and supports compliance excellence in medical device evaluations.




