Introduction
In the realm of medical device regulation, Clinical Evaluation Reports (CERs) serve as a cornerstone for ensuring the safety and efficacy of innovative technologies. Mandated by the Medical Device Regulation (MDR), these comprehensive documents not only validate clinical data but also play a crucial role in regulatory compliance and patient safety. As the landscape of medical devices evolves, the importance of well-structured CERs becomes increasingly apparent, necessitating a thorough understanding of their components, challenges, and the implications of regulatory frameworks.
This article delves into the intricacies of CERs, exploring their significance, key structural elements, compliance requirements under the MDR, and the future trends that will shape clinical evaluations in the medical device industry. By examining these facets, stakeholders can better navigate the complexities of clinical evaluations, ultimately leading to enhanced patient outcomes and market access.
Understanding Clinical Evaluation Reports: Definition and Significance
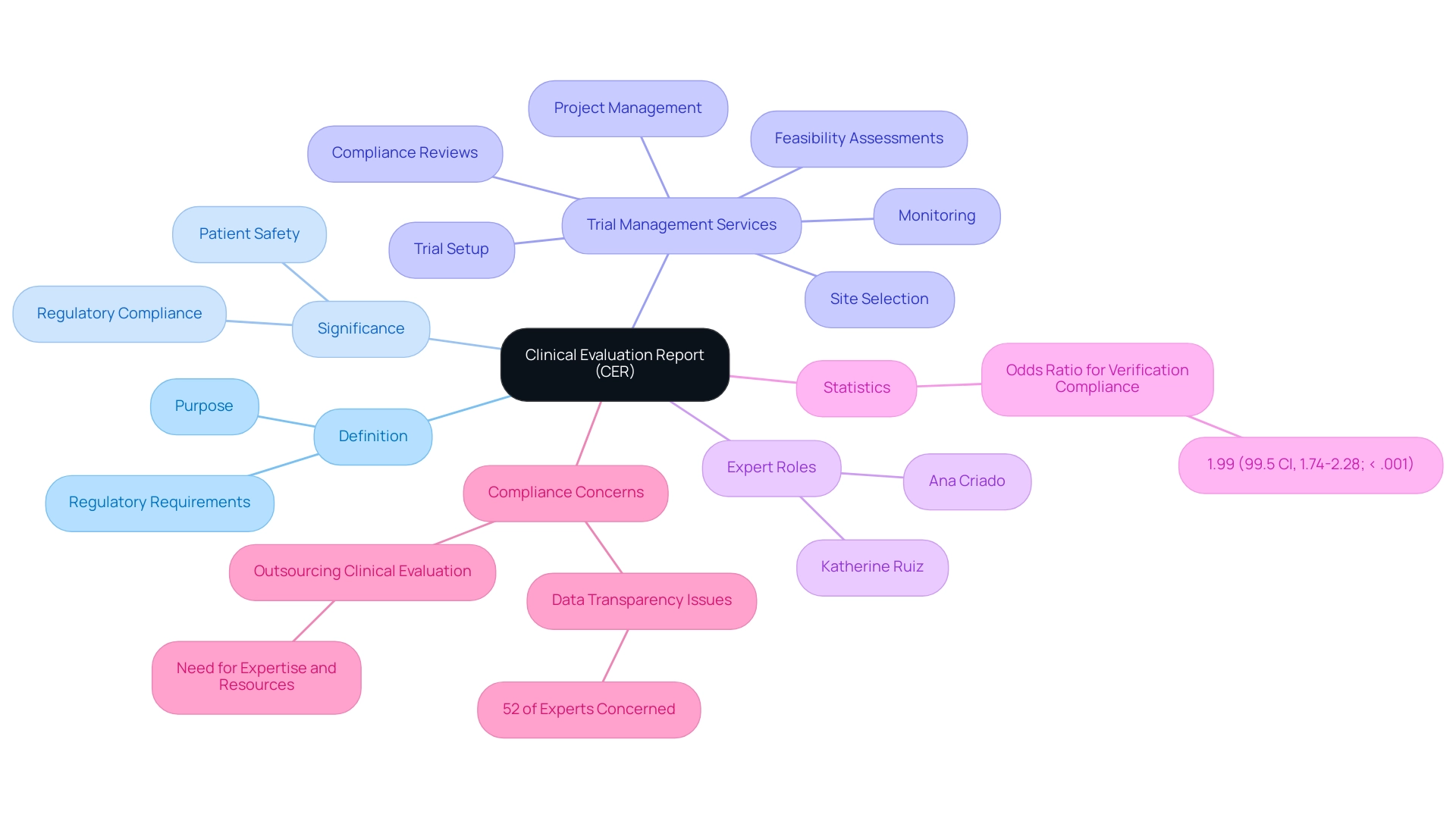
A clinical evaluation report template MDR is a crucial document required by the Medical Product Regulation (MDR), which is responsible for assessing data pertinent to a medical instrument. Its primary aim is to validate the safety and effectiveness of the apparatus through thorough research evidence. The significance of the clinical evaluation report template MDR extends beyond mere compliance; it plays a pivotal role in ensuring that medical devices adhere to regulatory standards, thereby safeguarding patient safety.
Thorough trial management services, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Project management
- Monitoring
are essential for the successful execution of these evaluations. By providing regulatory authorities with critical information, the clinical evaluation report template MDR facilitates the assessment of compliance with safety and performance criteria. Furthermore, specialists like Ana Criado, a Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices in Colombia, underscore the importance of expertise in the CER process.
Recent statistics indicate an odds ratio of 1.99 for verification compliance in applicable clinical trials, highlighting the necessity of thorough evaluations. As noted, 'Outsourcing your Clinical Evaluation means you should get the expertise and resources needed to complete it.' The inclusion of supplementary materials, such as detailed methods, analysis population flowcharts, and comprehensive reporting on study status, inventory, and serious and non-serious adverse events, enhances the comprehensiveness of the clinical evaluation report template MDR, ensuring that all relevant data is thoroughly documented.
In a landscape where 52% of compliance experts express concerns over data transparency with partners, the importance of a clinical evaluation report template MDR becomes even more pronounced, ensuring that products are not only compliant but also trustworthy for patient use. Consequently, the clinical evaluation report template MDR serves as a vital link in the regulatory submission process, aiding market access and improving health outcomes for patients, particularly within the framework established by entities like INVIMA, the Colombian National Food and Drug Surveillance Institute.
Key Components of a Clinical Evaluation Report: Structure and Requirements
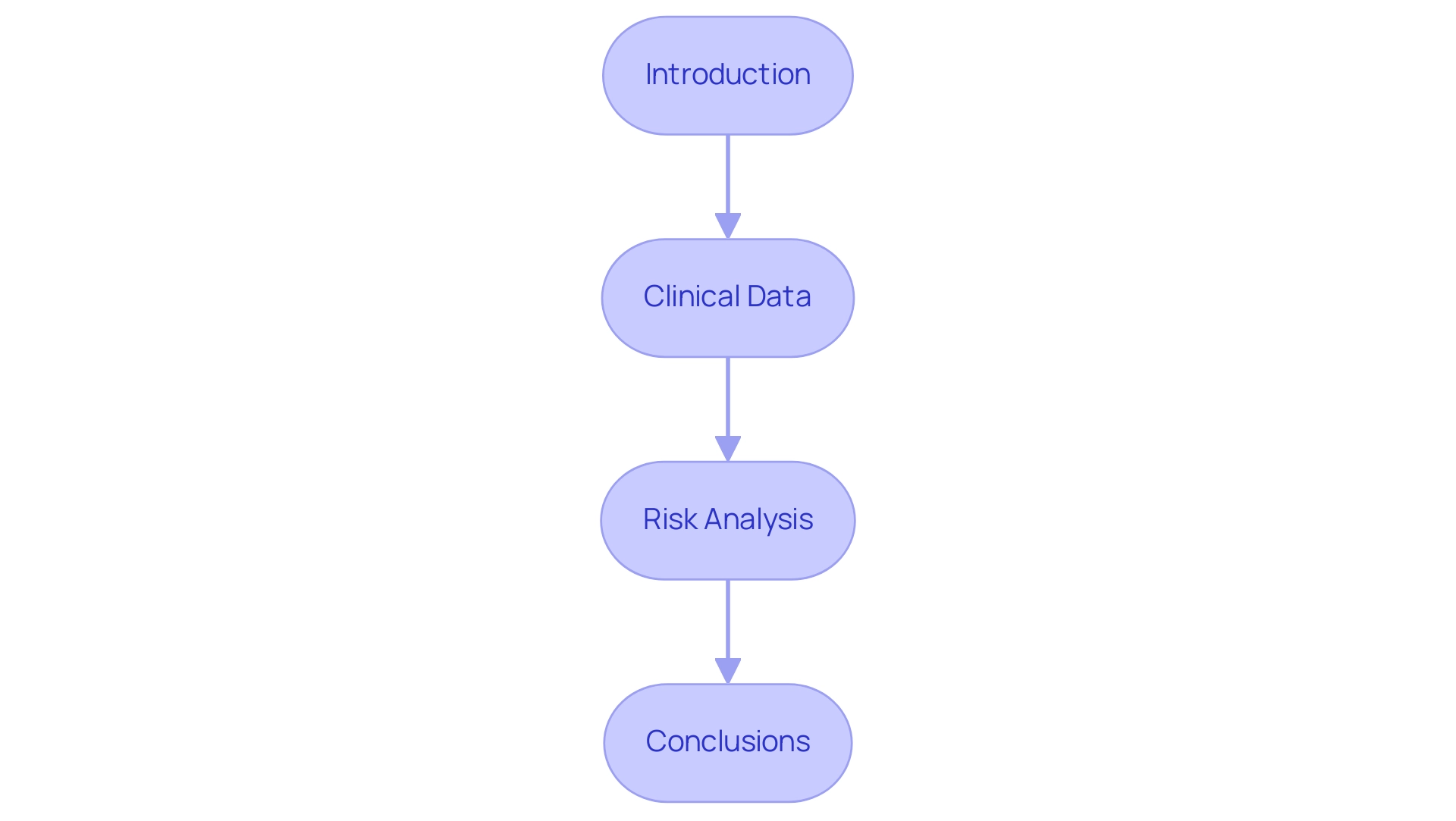
A Clinical Evaluation Report (CER) is essential for demonstrating the safety and effectiveness of a medical apparatus and comprises several critical components:
-
Introduction: This section establishes the groundwork by providing a brief summary of the apparatus, including its intended function and the medical context in which it will be utilized.
Equipment Description: Here, detailed information is provided about the medical equipment, covering aspects such as design specifications, materials used, and the underlying technology.
-
Clinical Data: This component entails a thorough review of clinical studies relevant to the apparatus, discussing methodologies employed, results obtained, and the conclusions drawn from the data. Such comprehensive data is pivotal in supporting claims of safety and effectiveness.
-
Risk Analysis: An in-depth assessment of potential risks associated with the apparatus is conducted in this section, addressing adverse events and outlining mitigation strategies to ensure patient safety.
-
Conclusions: The report culminates in a summary of findings, highlighting their implications for the product’s safety and performance.
Each component of the clinical evaluation report template mdr must strictly adhere to regulatory guidelines, particularly under the Medical Product Regulation (MDR), to ensure that the report is exhaustive and compliant with the latest requirements. Engaging with regulatory authorities throughout this process fosters open communication and can significantly expedite the approval process—as illustrated in case studies highlighting proactive engagement strategies. Furthermore, the statistic 'Join 200,000+ other medical device professionals' underscores the significance of CERs in the medical device industry, emphasizing the collective expertise that informs best practices.
Niki Price aptly notes that 'state of the art' refers to the best practices currently available in the marketplace, reinforcing the importance of aligning assessments with these standards. Additionally, employing an item-based approach for documentation can effectively navigate the regulatory landscape, ensuring that all essential elements are thoroughly addressed. Our services cover a variety of research activities including Early-Feasibility Assessments (EFA), First-In-Human Trials (FIH), Pilot Trials, Pivotal Trials, and Post-Market Follow-Up Evaluations (PMCF), which are essential to the evaluation process.
Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, emphasizes the importance of compliance and thorough documentation in achieving successful trial outcomes.
Regulatory Compliance: The Role of MDR in Clinical Evaluation Reports
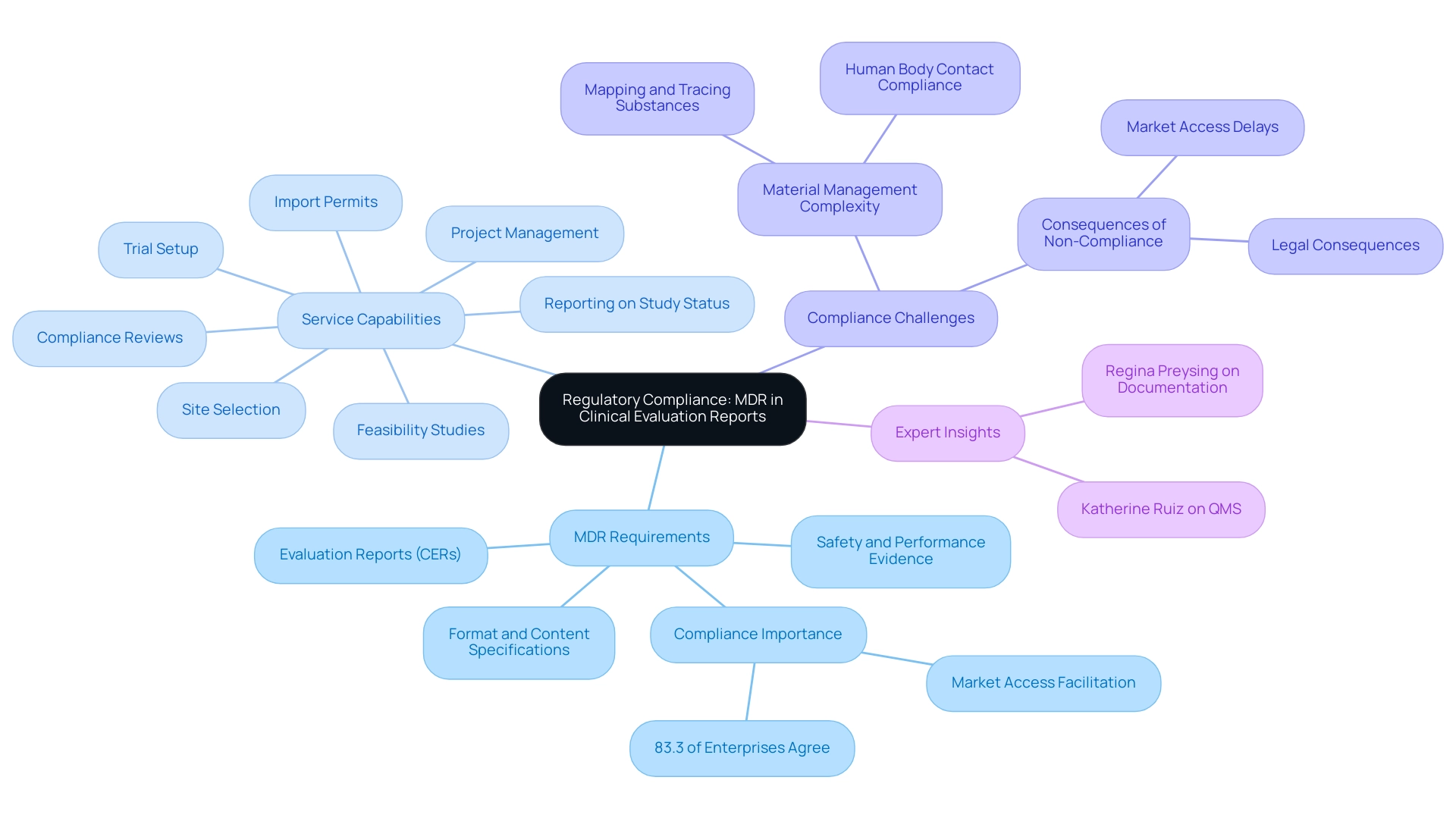
The Medical Device Regulation (MDR) establishes comprehensive requirements for Evaluation Reports (CERs), mandating that manufacturers present sufficient evidence to validate the safety and performance of their devices. Compliance with the MDR is essential, as it specifies the format, content, and level of detail necessary for a clinical evaluation report template mdr. In fact, 83.3% of large enterprises affirm that the MDR facilitates fair market access, emphasizing its pivotal role in the regulatory landscape.
Our service capabilities include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting on study status, inventory, and both serious and non-serious adverse events to ensure successful navigation of these regulatory requirements.
In preparing these reports, manufacturers must also consider the associated guidance documents from the European Commission regarding the clinical evaluation report template mdr for clinical evaluations. Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, emphasizes,
Having the right tools in place for QMS and documentation is a crucial success factor for the time to product marketing and thus for the business success of your company.
A pertinent example of compliance challenges can be seen in the case study titled 'Challenges in Material Management for Medical Devices,' which highlights the complexity of materials and specifications used in manufacturing. This complexity necessitates reliable mapping and tracing of substances, including those not present in the final product but used during manufacturing. Effective tracking and tracing of all substances is crucial for compliance with MDR, particularly in relation to human body contact and the approval process.
Non-compliance with these regulations can lead to considerable delays in market access and potential legal consequences. Therefore, it is essential for companies to strictly follow MDR guidelines when creating their clinical evaluation report template mdr, especially as changes in the Medical Device Regulation progress in 2024, including updates on evidence requirements and increased scrutiny on compliance processes.
Challenges in Clinical Evaluation: Data Collection and Analysis
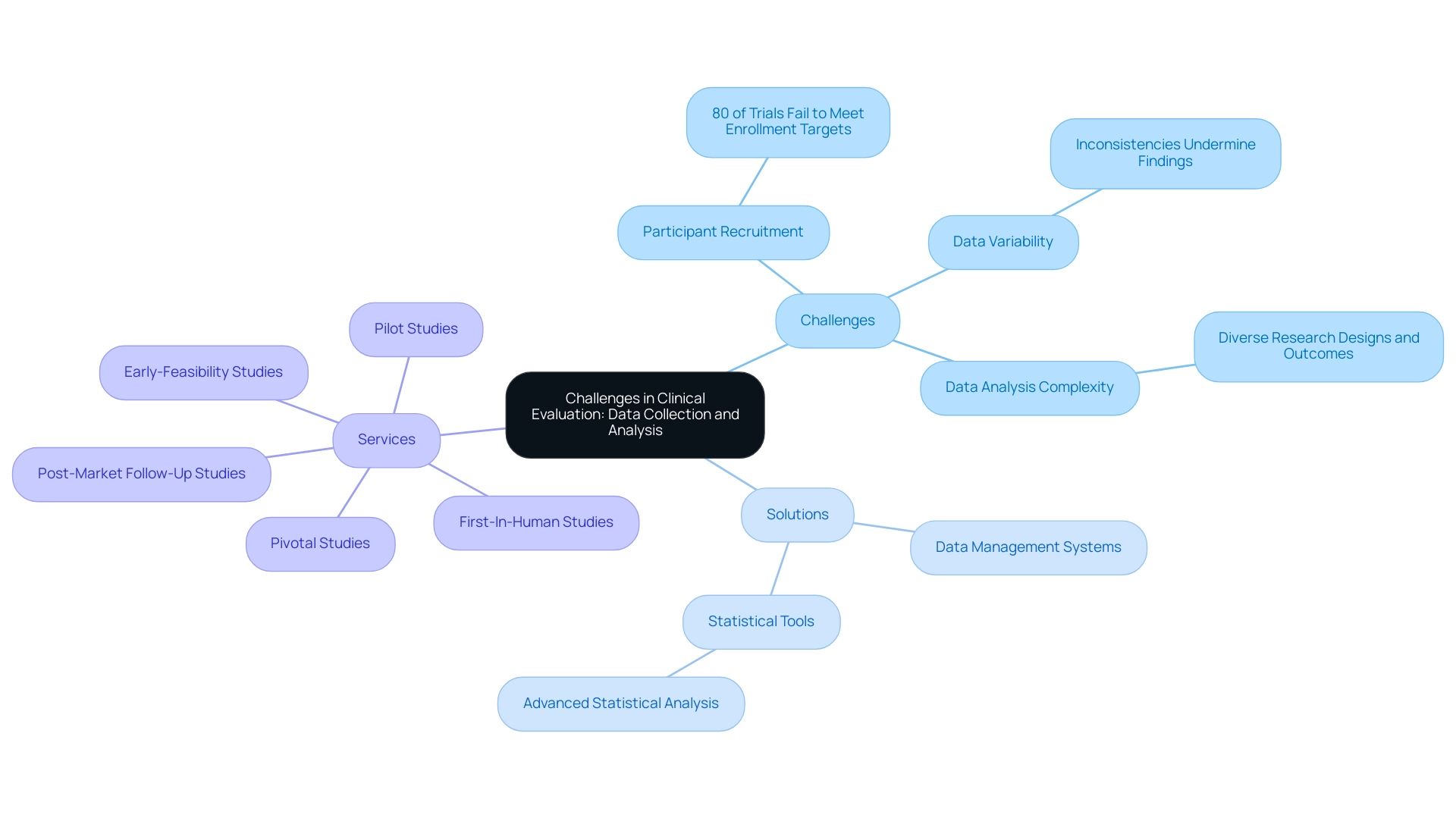
Data gathering and analysis serve as essential elements of the evaluation process, yet they are filled with challenges. One significant obstacle is the recruitment of suitable study participants, with studies indicating that approximately 80% of trials fail to meet their enrollment targets, which can severely impact the validity of the research. Variability in medical data further complicates the landscape, as inconsistencies can undermine the integrity of findings.
As highlighted by Altman and Bland, 'Absence of evidence is not evidence of absence,' stressing that the lack of data should be interpreted with caution, particularly when evaluating the reliability of assessments. Furthermore, the complexity of analyzing data to yield meaningful insights is heightened by various research designs and outcomes. Recent literature, including a review by Bewick et al. on receiver operating characteristic curves, highlights the intricacies involved in data interpretation.
To navigate these challenges, it is imperative to implement comprehensive data management systems alongside meticulous planning and protocol development. Utilizing advanced statistical analysis tools can significantly enhance the reliability of findings, thereby fortifying the clinical evaluation report template mdr.
Furthermore, bioaccess® provides accelerated trial management services in Latin America, specializing in:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies
Our services encompass trial setup, start-up, and approval processes, ensuring compliance with regulatory requirements. The adoption of routine pilot experiments before full-scale trials has emerged as an effective strategy to enhance research designs and reduce methodological weaknesses in medical research.
Additionally, the application of AI and advanced automation in clinical trials, as illustrated in the case study 'Applied AI and Advanced Automation in Clinical Trials,' demonstrates how these technologies can enhance data collection and analysis, ultimately leading to more reliable clinical evaluations. To learn more about how we can assist you, BOOK A MEETING with our team.
Future Trends in Clinical Evaluation Reports: Best Practices and Innovations
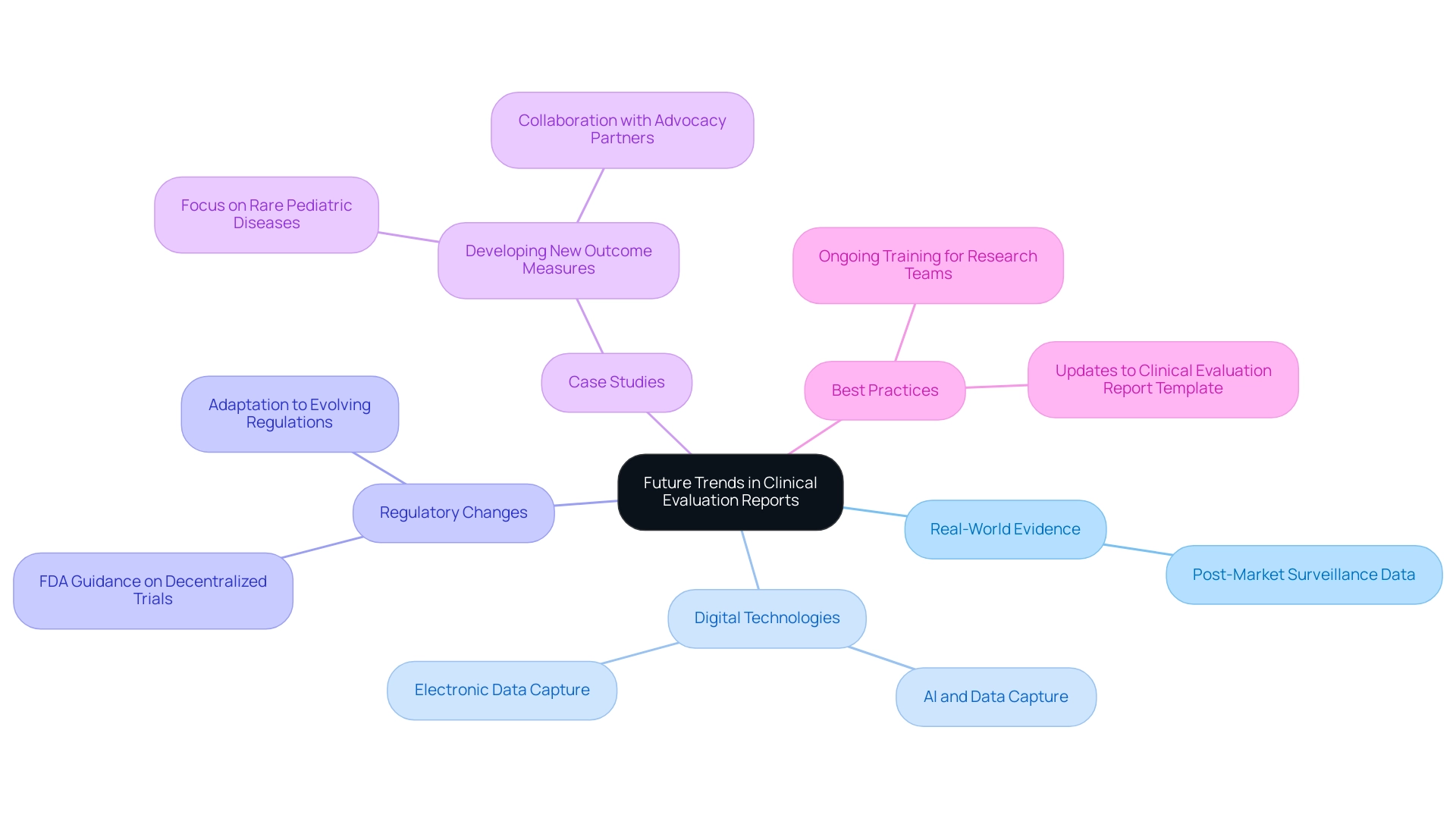
The medical equipment industry is undergoing significant transformation, which is driving the development of a clinical evaluation report template mdr for Clinical Evaluation Reports (CERs). Future trends suggest an increasing reliance on real-world evidence and post-market surveillance data, which are crucial for gaining insights into the long-term performance of medical devices. This shift is supported by the recent success of Phase III trials, which saw rates climb to 66%, surpassing the pre-pandemic average of 56%.
As mentioned by Sonia Abrol, Senior Vice President at WCG Velos, 'The COVID-19 pandemic made many stakeholders in research reassess their technology infrastructure,' emphasizing the urgent need for innovative practices. The integration of digital technologies, such as electronic data capture and artificial intelligence, stands to streamline both data collection and analysis processes, enhancing efficiency. Furthermore, maintaining an agile approach to regulatory compliance is vital as regulations evolve alongside scientific advancements.
Recent FDA guidance on decentralized medical trials and electronic systems underscores the importance of adapting to these regulatory changes. By utilizing specialized knowledge and tailored solutions offered through 14 therapeutic centers of excellence, including oncology and neurology, stakeholders can improve their evaluation processes. Bioaccess®, with more than 20 years of experience in Medtech, excels in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies, ensuring comprehensive trial management services.
Furthermore, case analyses, such as the development of new outcome measures for rare pediatric and CNS diseases, illustrate the real-world impact of these innovations on local economies, fostering job creation, economic growth, and healthcare improvement. By adopting best practices, including ongoing training for research teams and consistent updates to the clinical evaluation report template mdr, stakeholders can ensure that evaluations remain relevant, robust, and compliant with emerging standards. The flexibility required in managing these clinical trials is also a key component of bioaccess®'s approach, ensuring that each study can adapt to the unique challenges it may encounter.
Conclusion
The significance of Clinical Evaluation Reports (CERs) in the medical device regulatory landscape cannot be overstated. These essential documents, mandated by the Medical Device Regulation (MDR), serve as a critical foundation for validating the safety and efficacy of medical devices through rigorous clinical evidence. By systematically detailing the device's intended purpose, clinical data, risk analysis, and compliance with regulatory standards, CERs ensure that manufacturers meet the necessary requirements, ultimately safeguarding patient safety.
Navigating the complexities of clinical evaluations presents numerous challenges, particularly in data collection and analysis. Engaging the right expertise and adopting innovative practices, including advanced statistical tools and digital technologies, can mitigate these challenges. Furthermore, the incorporation of real-world evidence and post-market surveillance data is poised to redefine best practices in CER preparation, allowing stakeholders to gain deeper insights into the long-term performance of medical devices.
Looking ahead, the medical device industry is set to evolve with ongoing advancements in regulatory frameworks and technologies. Emphasizing compliance and thorough documentation will be vital for successful market access and improved patient outcomes. By embracing these changes and leveraging specialized resources, stakeholders can enhance their clinical evaluation processes, ensuring that CERs remain robust, relevant, and aligned with emerging standards. The future of medical device regulation hinges on the commitment to excellence in clinical evaluations, reinforcing the critical role that CERs play in delivering safe and effective healthcare solutions.
Frequently Asked Questions
What is a clinical evaluation report template MDR?
A clinical evaluation report template MDR is a crucial document required by the Medical Product Regulation (MDR) that assesses data related to a medical instrument, validating its safety and effectiveness through thorough research evidence.
Why is the clinical evaluation report template MDR important?
It ensures compliance with regulatory standards, safeguarding patient safety, and plays a vital role in the regulatory submission process, aiding market access and improving health outcomes for patients.
What services are involved in trial management for clinical evaluations?
Essential trial management services include feasibility assessments, site selection, compliance reviews, trial setup, project management, and monitoring.
Who are some experts that highlight the importance of expertise in the CER process?
Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices in Colombia, emphasize the importance of expertise in the clinical evaluation report process.
What recent statistics highlight the necessity of thorough evaluations in clinical trials?
Recent statistics indicate an odds ratio of 1.99 for verification compliance in applicable clinical trials.
What supplementary materials enhance the clinical evaluation report template MDR?
Supplementary materials may include detailed methods, analysis population flowcharts, and comprehensive reporting on study status, inventory, and adverse events.
What concerns do compliance experts have regarding data transparency?
52% of compliance experts express concerns over data transparency with partners, underscoring the importance of a clinical evaluation report template MDR for ensuring compliance and trustworthiness.
What are the key components of a Clinical Evaluation Report (CER)?
The key components include an introduction, clinical data review, risk analysis, and conclusions, all of which must adhere to regulatory guidelines.
How does engaging with regulatory authorities impact the approval process?
Engaging with regulatory authorities fosters open communication, which can significantly expedite the approval process.
What types of research activities are covered in the evaluation process?
Research activities include Early-Feasibility Assessments (EFA), First-In-Human Trials (FIH), Pilot Trials, Pivotal Trials, and Post-Market Follow-Up Evaluations (PMCF).

