Introduction
In the realm of clinical research, the importance of establishing robust Standard Operating Procedures (SOPs) cannot be overstated. These meticulously crafted documents serve as the backbone for conducting clinical evaluations, ensuring that research activities adhere to regulatory standards and best practices.
With the increasing complexity of clinical trials and the evolving landscape of medical device regulation, organizations must prioritize the development and implementation of comprehensive SOP templates. These templates not only promote consistency and compliance but also enhance the quality and reliability of research outcomes.
From defining roles and responsibilities to outlining procedures for data collection and quality assurance, a well-structured SOP template is essential for guiding research teams through the intricacies of clinical evaluations. As the industry continues to adapt to new challenges, the role of SOPs in safeguarding ethical considerations and maintaining research integrity becomes ever more critical.
Defining the Clinical Evaluation SOP Template
A clinical evaluation SOP template for medical assessments is a carefully organized document that outlines the processes and guidelines essential for performing assessments within research settings. This clinical evaluation SOP template is essential for ensuring consistency, adherence, and the overall quality of research activities in the health sector. By establishing standardized procedures, it facilitates adherence to regulatory requirements and best practices, thereby enhancing the reliability of research outcomes.
According to PhRMA guidelines, the SOP must address critical aspects such as ethics, data integrity, and safety reporting, ensuring that all evaluations uphold the highest standards of research integrity. Additionally, our extensive clinical trial management services encompass:
- Feasibility studies
- Site selection
- Regulatory reviews
- Trial setup
- Import permits
- Project management
- Reporting (study status, inventory, serious and non-serious adverse events)
- Monitoring
All aimed at effectively supporting these SOPs. Typically, a well-crafted clinical evaluation SOP template includes critical sections, such as:
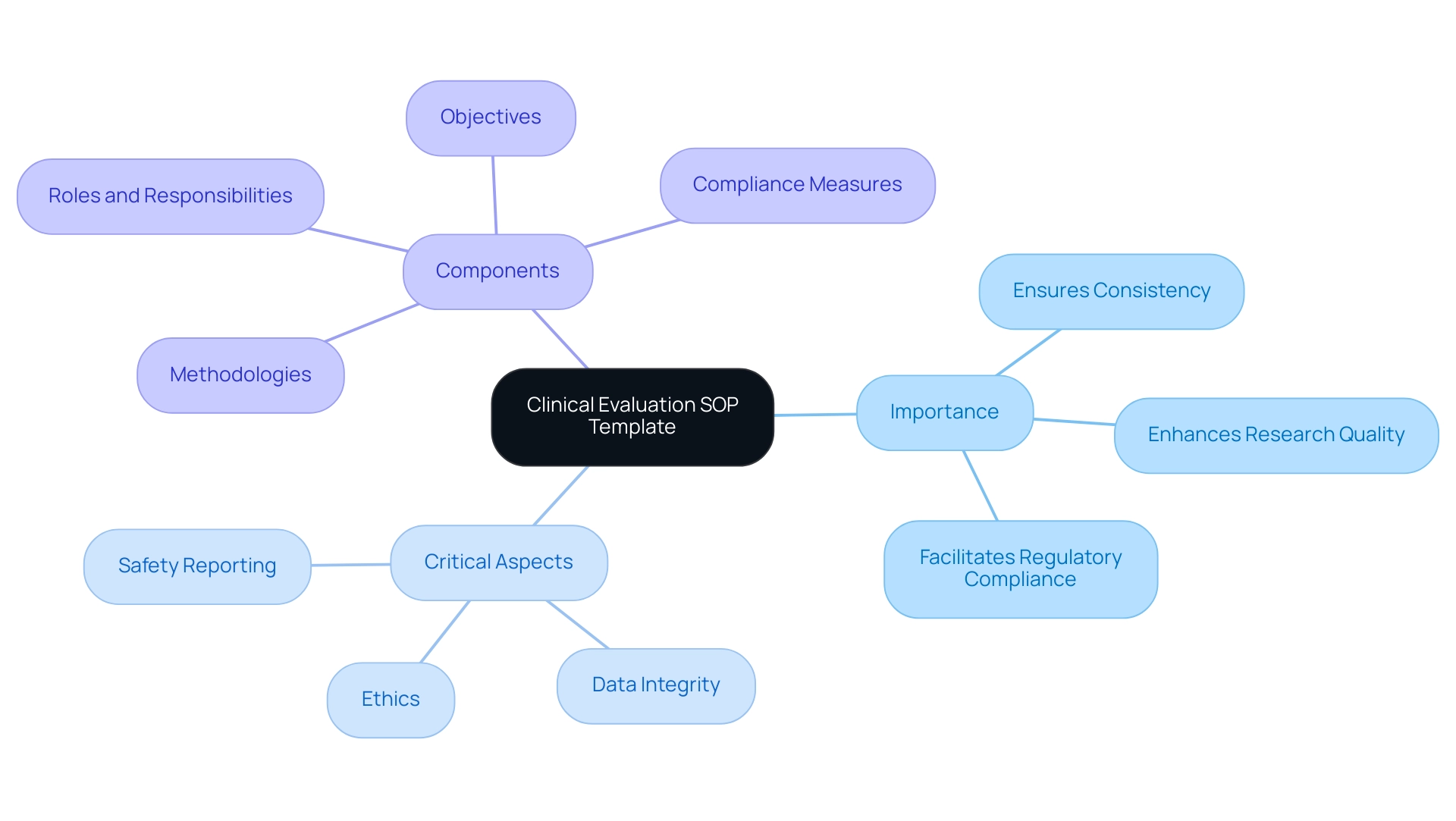
- Objectives
- Methodologies
- Roles and responsibilities
- Compliance measures
These components collectively serve to guide the research team in executing evaluations with both effectiveness and efficiency. As Maria Nyåkern, Ph.D., founder of AKRN Scientific Consulting, emphasizes, she has extensive ‘hands-on’ experience in establishing and overseeing numerous research investigations, which highlights the significance of having strong SOPs in place. In the context of Colombia, understanding the role of INVIMA as a Level 4 health authority by PAHO/WHO is crucial for ensuring adherence in medical device oversight.
As the landscape of medical research continues to evolve, particularly with the integration of digital biomarkers, the importance of having a clinical evaluation SOP template will only grow, ensuring that safety and ethical considerations remain at the forefront of investigations. Maria Nyåkern's knowledge, especially in health assessments according to EU regulations, exemplifies the practical application of these SOPs, reinforcing their essential role in enhancing the quality and adherence of medical research.
Key Components of a Clinical Evaluation SOP Template
A well-crafted Clinical Evaluation SOP Template encompasses several essential components that ensure clarity and compliance throughout the clinical research process:
- Purpose and Scope: This section clearly outlines the aims of the SOP and the specific research activities it includes, highlighting the significance of a defined framework for assessments, which aligns with best practices in the field.
- Definitions and Acronyms: To avoid ambiguity, this part provides precise definitions of terminology used within the SOP, promoting a common understanding among all stakeholders involved.
- Roles and Responsibilities: Here, the duties of team members involved in the medical assessment process are outlined, ensuring accountability and clarity in task assignments.
- Procedures: This essential element encompasses comprehensive, step-by-step guidelines for performing medical assessments, addressing data gathering techniques and appraisal standards that conform to recognized best practices. It also encompasses the processes of feasibility studies and site selection, which are crucial for effective trial management.
- Compliance and Quality Assurance: This section describes the mechanisms in place to guarantee adherence to regulatory standards, including oversight by INVIMA, Colombia's national authority classified as a Level 4 health authority by PAHO/WHO. Quality control procedures are essential for preserving the integrity of the assessment, ensuring that trials meet the required legal and ethical standards.
- Documentation and Record Keeping: Accurate record-keeping is essential, and this component specifies the requirements for maintaining thorough documentation of assessments, which is vital for audits and inspections. This encompasses thorough reporting systems for study status, inventory, and adverse events, as emphasized by the registered trials unit survey, which garnered 46 responses, highlighting the necessity for a clinical evaluation SOP template in the context of medical assessments.
In the context of identifying pertinent medical information, the case study titled 'Identifying Data for Medical Assessment' illustrates the necessity of accurately collecting both data generated by the company and external information, which is fundamental for fulfilling the requirements of the medical assessment plan.
In line with regulatory expectations, the MEDDEV 2.7/1 Rev. 4 guideline states, > The manufacturer should define and justify the frequency of CER updates <, emphasizing the continuous aspect of assessment. Moreover, the insights offered by Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, emphasize the significance of strict adherence to these guidelines.
Moreover, the guidelines detailed in the article 'Guidelines for the Content of Statistical Analysis Plans in Clinical Trials' (DOI: 10.1001/jama.2017.18556) offer additional insights into the significance of thorough statistical planning in medical assessments. By incorporating these essential elements, such as thorough project management and reporting processes, organizations can guarantee adherence and improve the quality of their assessment procedures.
The Importance of Clinical Evaluation SOPs in Research Compliance
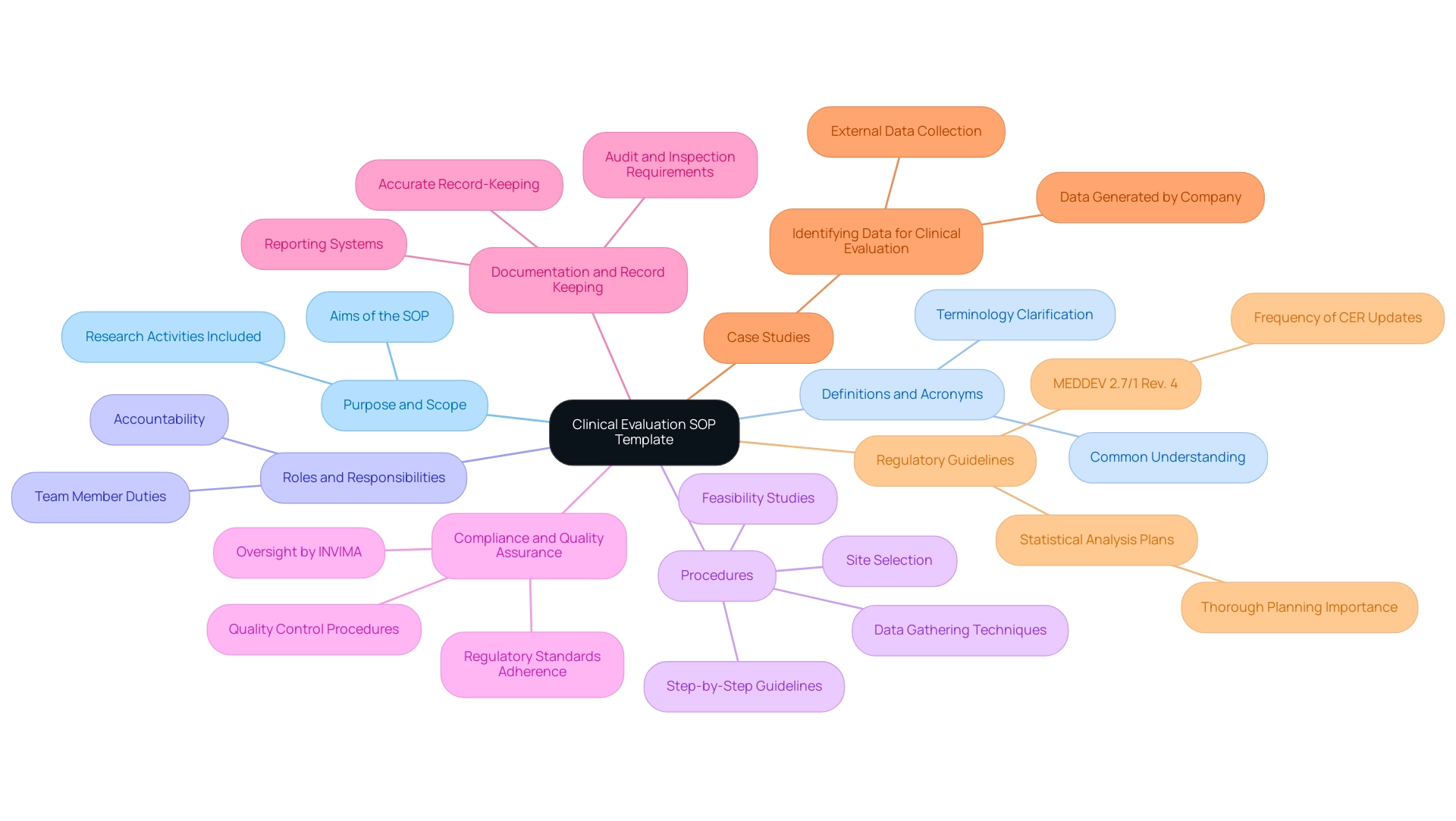
The clinical evaluation SOP template is essential for guaranteeing adherence to regulatory standards set by authorities such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Our comprehensive trial management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Review and feedback on study documents to comply with country requirements
We also assist in import permits and the nationalization of investigational devices, offering researchers organized guidelines intended to reduce risks linked to trials.
This significantly reduces the likelihood of errors and enhances the reliability of the data collected. Moreover, adherence to these procedures fosters transparency and accountability, which are essential for sustaining public trust in research. In a landscape characterized by heightened regulatory scrutiny, effective SOPs, along with project management, monitoring, and reporting on serious and non-serious adverse events, act as crucial safeguards against potential pitfalls that could compromise the validity of research findings.
With recent data indicating that 94% of interventional research studies have successfully published their results, the significance of strong management systems becomes even more evident. Experts like Katherine Ruiz, a noted authority in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, emphasize that aligning global standards with local requirements is paramount for achieving reliable outcomes in clinical trials. As Wil Flanagan observes, 'Corrective and Preventive Action (CAPA) in Research' is an essential aspect of compliance, emphasizing the need for effective standard operating procedures.
Furthermore, the case study titled '5 Truths About Inducements in Research: Expert Guide 2024' emphasizes ethical considerations and best practices for improving participant engagement, further demonstrating the necessity of thorough Clinical Evaluation procedures. Thus, the continued focus on the implementation of procedures such as the clinical evaluation SOP template is not only a best practice but also a necessity in the evolving regulatory environment.
Best Practices for Developing Effective Clinical Evaluation SOPs
To create effective Clinical Evaluation Standard Operating Procedures, researchers should follow these best practices:
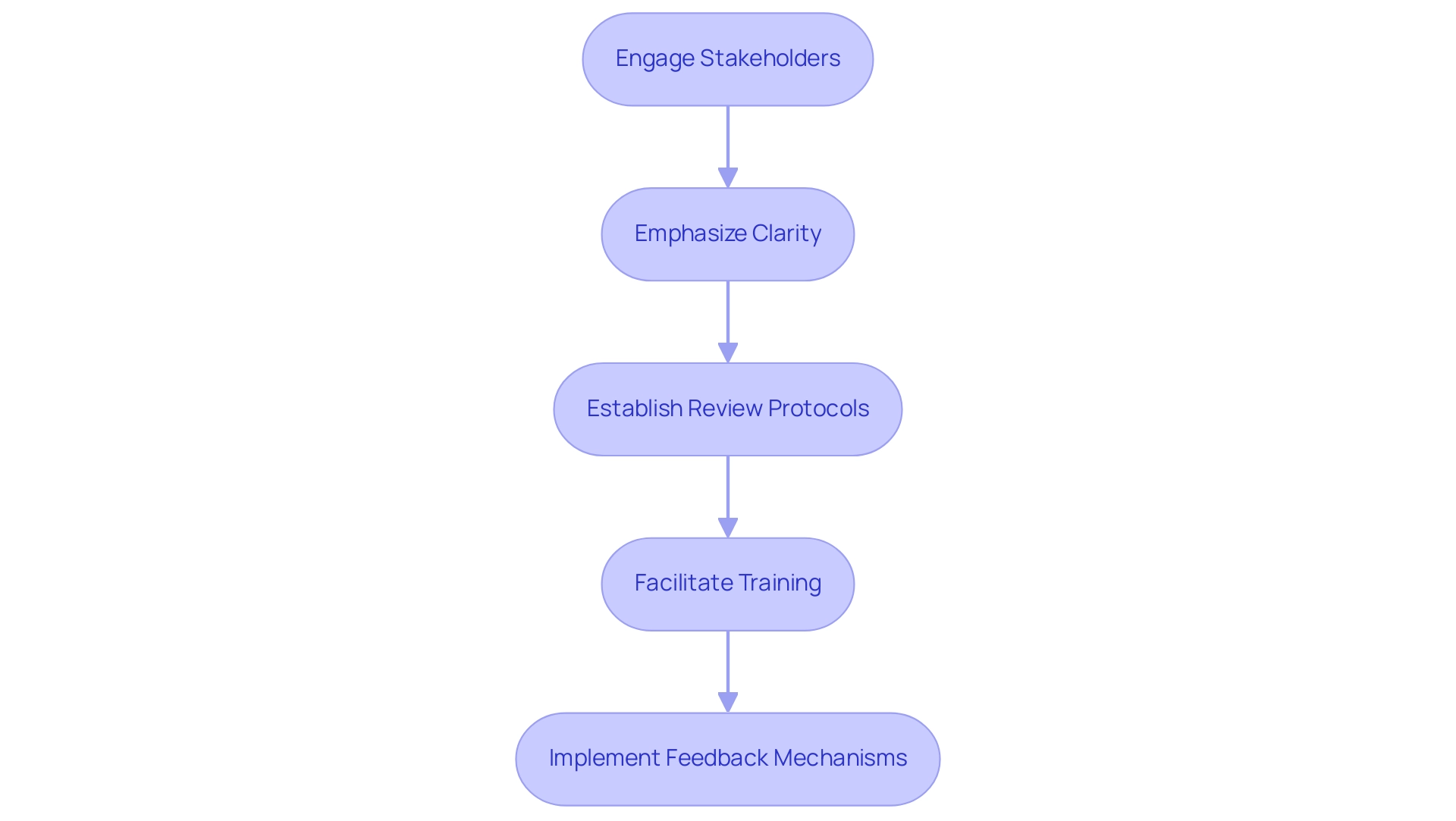
- Engage Stakeholders: Involving team members, regulatory experts, and other key stakeholders in the SOP development process enhances the comprehensiveness of the guidelines. This collaborative approach ensures that diverse perspectives are incorporated into the clinical evaluation sop template, facilitating smoother implementation and adherence. An initial scoping exercise with UKCRC CTU Statisticians revealed that while GCP training is valuable, its relevance to statistical roles could be improved through stakeholder engagement. This emphasizes the necessity of relevant training for stakeholders in their specific roles.
- Emphasize Clarity: Utilizing clear and concise language is essential to ensure that the standard operating procedures are easily comprehensible for all team members, regardless of their experience level. This accessibility promotes uniform understanding and adherence across the team. The training material, consisting of 114 slides divided into five modules, exemplifies how structured content can enhance clarity and comprehension.
- Establish Review Protocols: Regularly scheduled reviews and updates of the SOPs are crucial to maintaining compliance with evolving regulations, emerging best practices, and technological advancements. This proactive method helps protect the integrity of the medical assessment process. The guidelines for Statistical Analysis Plans (SAPs) illustrate the importance of establishing clear protocols and consensus among stakeholders to enhance transparency and reproducibility in research.
- Facilitate Training: Comprehensive training sessions are necessary to ensure that all team members are well-versed in the clinical evaluation sop template and understand their respective roles within the clinical evaluation framework. Proper training mitigates risks associated with non-compliance and enhances overall team effectiveness.
- Implement Feedback Mechanisms: A structured system for gathering feedback from users of the procedures is vital for continuous improvement. This feedback loop facilitates the identification of potential areas for enhancement, ensuring that the standard operating procedures remain relevant and practical in real-world applications.
Clinical Evaluation SOPs and Their Role in Medical Device Regulation
The clinical evaluation SOP template is foundational to the regulation of medical devices, providing a structured framework for assessing product safety and efficacy. Regulatory bodies require that producers conduct medical assessments as an essential part of the pre-market approval procedure. These procedures guarantee that assessments are carried out uniformly and in accordance with current guidelines, including the revised ISO 14155 standard for research investigations.
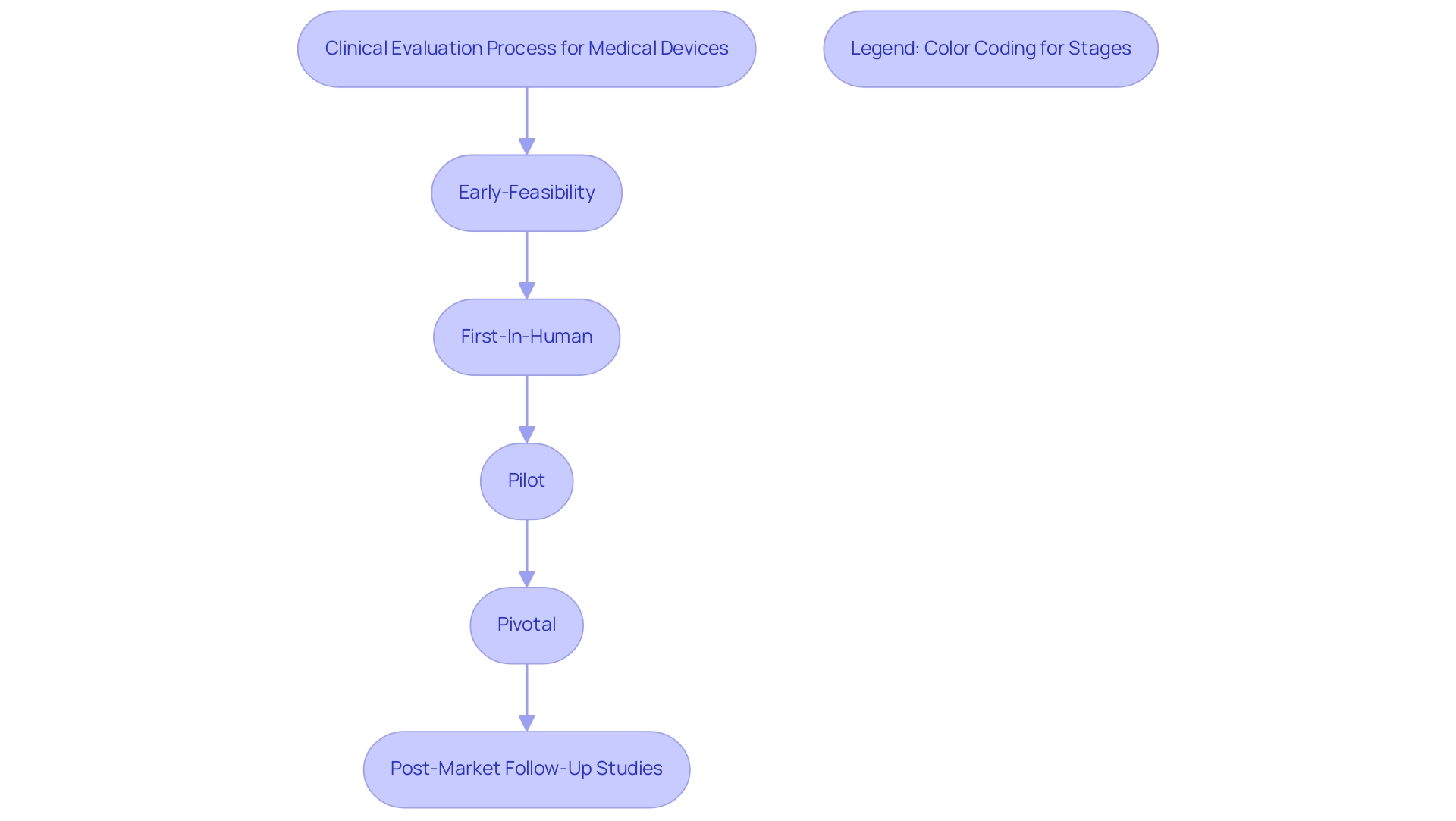
Compliance with these established SOPs not only reflects manufacturers' dedication to quality and regulatory adherence but also plays a pivotal role in securing market access and fostering consumer confidence. Bioaccess® utilizes over 20 years of expertise in managing comprehensive trial services in Latin America, including:
- Early-Feasibility
- First-In-Human
- Pilot
- Pivotal
- Post-Market Follow-Up Studies
This ensures a customized approach to meet diverse regulatory requirements. Our services encompass thorough compliance reviews and meticulous trial setup processes, which are essential for ensuring that all studies adhere to local regulations and international standards.
Significantly, Maria Nyåkern has overseen numerous medical studies involving tens of thousands of patients, highlighting the significance of these assessments in practical environments. As highlighted in MDCG 2020-6, the use of relatively simple, common, and stable designs has proven effective; these designs belong to device groups that exhibit well-known safety profiles and have not been associated with safety issues in the past. Furthermore, the case study on Mesh Term Relevance Screening demonstrates how evaluating the relevance of Mesh terms extracted from seed publications can refine search strategies for clinical data retrieval, further supporting the importance of rigorous clinical evaluation in the regulatory framework.
By prioritizing the clinical evaluation SOP template and navigating the complexities of compliance reviews and trial setups, manufacturers contribute to the overall safety assessments critical for pre-market approval, thereby enhancing the integrity of the medical device industry.
Conclusion
Establishing robust Clinical Evaluation Standard Operating Procedures (SOPs) is essential for ensuring the integrity, compliance, and quality of clinical research. These meticulously structured documents not only define the processes and guidelines necessary for conducting evaluations but also promote consistency and adherence to regulatory standards. By clearly outlining roles, responsibilities, and detailed procedures, SOPs serve as a critical framework that guides research teams through the complexities of clinical trials, ultimately enhancing the reliability of research outcomes.
The key components of a well-crafted SOP template—including purpose, definitions, compliance measures, and documentation standards—are instrumental in fostering accountability and transparency within research environments. As regulatory scrutiny intensifies, the importance of adhering to these SOPs cannot be overstated. They act as safeguards that mitigate risks, ensuring that ethical considerations are prioritized and that the integrity of the research is maintained. Furthermore, the alignment of global standards with local regulations is crucial for achieving reliable results and sustaining public trust in clinical research.
In an evolving landscape where the integration of new technologies and methodologies is constant, continuous improvement and training are paramount. Engaging stakeholders in the SOP development process, emphasizing clarity, and implementing feedback mechanisms will enhance the effectiveness of these procedures. By prioritizing the development and implementation of comprehensive SOPs, organizations not only comply with regulatory requirements but also contribute to the advancement of clinical research, ultimately benefiting the safety and efficacy of medical devices and treatments.
Frequently Asked Questions
What is a clinical evaluation SOP template?
A clinical evaluation SOP template is a structured document that outlines the processes and guidelines necessary for conducting medical assessments in research settings, ensuring consistency, adherence to regulatory requirements, and quality in research activities.
Why is a clinical evaluation SOP template important?
It is essential for maintaining standardized procedures that enhance the reliability of research outcomes, uphold ethical standards, and ensure data integrity and safety reporting.
What critical aspects must a clinical evaluation SOP address according to PhRMA guidelines?
It must address ethics, data integrity, and safety reporting to ensure that all evaluations meet high standards of research integrity.
What are some of the services included in clinical trial management?
Services include feasibility studies, site selection, regulatory reviews, trial setup, import permits, project management, reporting on study status and adverse events, and monitoring.
What are the key components of a well-crafted clinical evaluation SOP template?
Key components include: 1. Objectives 2. Methodologies 3. Roles and responsibilities 4. Compliance measures.
What does the "Purpose and Scope" section of the SOP entail?
It outlines the aims of the SOP and the specific research activities it covers, establishing a defined framework for assessments aligned with best practices.
How does the SOP ensure clarity and accountability among team members?
By clearly defining roles and responsibilities, the SOP promotes accountability and clarity in task assignments among team members involved in the medical assessment process.
What is included in the "Procedures" section of the SOP?
This section provides comprehensive, step-by-step guidelines for performing medical assessments, including data gathering techniques and appraisal standards, as well as processes for feasibility studies and site selection.
How does the SOP address compliance and quality assurance?
It describes mechanisms for adherence to regulatory standards, including oversight by INVIMA, and outlines quality control procedures to preserve the integrity of assessments.
Why is documentation and record keeping important in the SOP?
Accurate record-keeping is vital for audits and inspections, ensuring thorough documentation of assessments, study status, inventory, and adverse events.
What does the case study "Identifying Data for Medical Assessment" illustrate?
It highlights the necessity of accurately collecting both internal and external data to fulfill the requirements of the medical assessment plan.
What is the significance of the MEDDEV 2.7/1 Rev. 4 guideline in the context of the SOP?
It emphasizes that manufacturers should define and justify the frequency of Clinical Evaluation Report (CER) updates, indicating the ongoing nature of assessments.
How do statistical analysis plans relate to clinical assessments?
Thorough statistical planning is crucial for ensuring the quality of medical assessments, as highlighted in related guidelines for clinical trials.




