Introduction
Clinical summary reports serve as the cornerstone of effective communication in clinical trials, encapsulating the intricate details of research findings for a diverse audience. As the landscape of medical technology continues to evolve, particularly in regions like Latin America, the importance of these reports cannot be overstated.
They not only provide a comprehensive overview of trial objectives, methodologies, and outcomes but also address the unique challenges faced by stakeholders, including regulatory bodies and sponsors. With the anticipated changes in reporting standards on the horizon, the role of clinical summary reports in enhancing transparency and fostering collaboration becomes increasingly critical.
This article delves into the essential components, purposes, and best practices for crafting these reports, ensuring that they meet the rigorous demands of the clinical research community while facilitating informed decision-making and advancing medical knowledge.
Defining the Clinical Summary Report: An Overview
A clinical summary report is a crucial text that encapsulates the results of a trial, providing a comprehensive overview of its objectives, methodology, findings, and conclusions. This clinical summary report is indispensable for stakeholders—including researchers, regulatory bodies, and sponsors—because it promotes a shared understanding of research outcomes, particularly regarding the challenges faced by Medtech companies in Latin America. With challenges like language obstacles and scattered resources, the clarity and detail offered in the clinical summary report are increasingly essential for ensuring effective communication and collaboration.
Bioaccess®, as a leading CRO in Latin America, plays a crucial role in facilitating this process by ensuring that study results are not only accurate but also actionable. Specifically, bioaccess® streamlines regulatory approval processes and enhances subject recruitment strategies, addressing the key challenges faced by Medtech startups. The organized layout of the clinical summary report highlights essential data and insights, rendering the information both reachable and applicable for decision-making procedures in medical research.
As the environment of medical study reporting standards develops, especially with expected alterations in 2024, the clinical summary report will improve transparency and accountability in research, promoting trust among stakeholders. This necessity is reflected in the increasing acknowledgment of thorough reporting standards, as mentioned by Gaunt P. in 'Early phase research extension to guidelines for the content of statistical analysis plans' (BMJ). Moreover, the case study titled 'Overview of Comments on Draft ICH E9(R1) Addendum' serves as a clinical summary report that emphasizes the significance of aligning research objectives and treatment effect descriptions to ensure improved planning and execution of studies, highlighting the urgency of closing the gap between innovation and implementation.
Key Components of a Clinical Summary Report
A comprehensive clinical summary report is essential for conveying study findings effectively, especially in the context of accelerated medical device clinical trials managed by bioaccess®. The key components include:
- Title Page: This section clearly identifies the report and the specific study it summarizes, providing essential context from the outset.
- Executive Summary: Serving as a snapshot of the entire study, this overview encapsulates the objectives, methods, and key findings, allowing readers to quickly grasp the essence of the research.
- Introduction: Here, the background of the study is outlined, including the research question and objectives, setting the stage for the subsequent sections. This is especially vital considering the high stakes of Early-Feasibility and First-In-Human studies.
- Methods: This essential component outlines the study design, participant selection criteria, and collection processes, ensuring transparency and reproducibility. Event rates are determined as the event count divided by the period of time, which adds a quantitative aspect to the discussion of information presentation.
- Results: Findings are presented in this section, often complemented by tables and figures to enhance clarity and facilitate understanding of the information.
- Discussion: In this part, interpretations of the results are articulated, discussing their implications and relevance to the field, and providing a platform for further exploration. The integrity of the research is emphasized by the statement from Matthew P. Smeltzer:
The author(s) received no financial support for the research, authorship, and/or publication of this article,
highlighting the significance of transparency in medical research.
- Conclusion: This section briefly summarizes the key takeaways from the study, reinforcing the main messages.
- References: A list of all sources cited throughout the document is included here, ensuring proper attribution and allowing for further investigation by interested readers.
Each of these components is essential for a comprehensive and informative clinical summary report, which helps to ensure that the findings are communicated effectively. Additionally, action buttons are increasingly being incorporated into documents, enabling users to drill down into data and view summary statistics for selected points from the volcano plot, enhancing user interaction and data presentation. A case study titled 'Binary Data Analysis' illustrates these principles by examining binary outcome variables and utilizing logistic regression methods, ultimately demonstrating how to estimate proportions and assess associations between treatment groups.
By utilizing the knowledge and tailored method of bioaccess®, which encompasses services such as Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, you guarantee that your research endeavors are not only compliant but also set for success in a competitive environment.
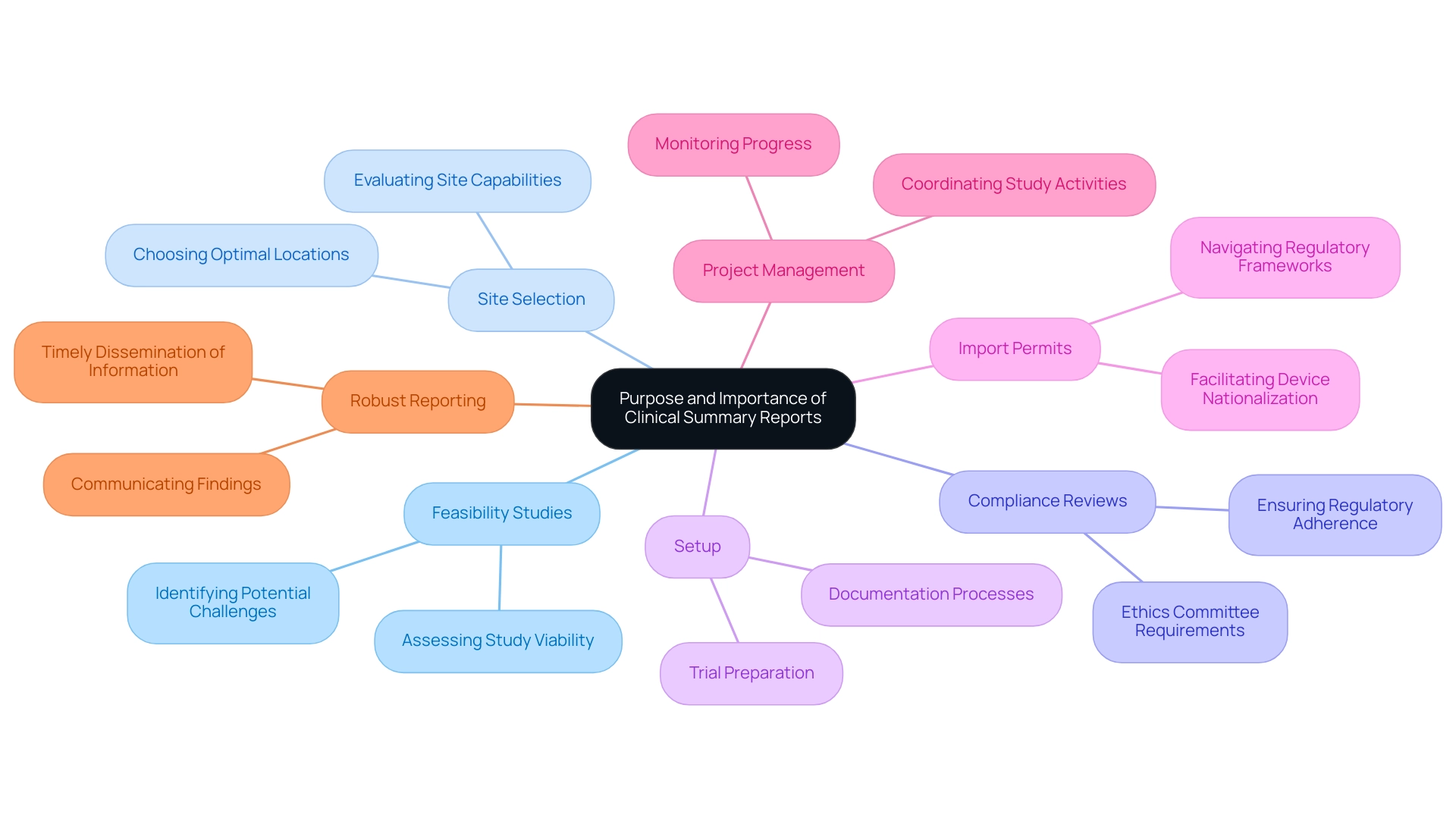
The Purpose and Importance of Clinical Summary Reports
Summary documents are essential in the realm of studies, serving multiple vital roles. Our comprehensive clinical study management services include:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Robust reporting
These reports primarily serve to communicate findings to stakeholders, including regulatory agencies, which mandate transparent reporting of trial results as part of the approval process.
Such transparency is essential; for instance, the committee recommends that information packages for abandoned studies be shared within 18 months. This timeline not only emphasizes the necessity for timely dissemination of information but also highlights the importance of transparency in ensuring that stakeholders have access to pertinent information. Furthermore, these documents play a pivotal role in disseminating medical knowledge, enabling the evaluation and replication of research findings by other researchers.
The case analysis regarding the use of research reports (Cars) illustrates this; twelve respondents acquired Cars, with nine employing this information to integrate unpublished studies into their meta-analyses, thereby significantly improving the quality of their research results. As expressed by O'Rourke and Forster, sponsors and lead researchers will ultimately decide the distribution of information from studies initiated before the new guidelines on an individual basis, reflecting current trends in information sharing and openness. This situation highlights the wider initiative to enhance access to research information, which not only enhances the societal advantages of studies but also fosters informed choices in patient care and upcoming research paths.
Furthermore, the committee suggests releasing particular categories of research data at various timepoints in the research lifecycle, further strengthening the organized method to data sharing. By ensuring that results are presented clearly and comprehensively, the clinical summary report contributes significantly to the integrity and advancement of medical research.
Furthermore, our services encompass comprehensive reporting on study status, inventory management, and the documentation of serious and non-serious adverse events, as well as compliance with the requirements established by the ethics committee and health ministry during the setup process.
The nationalization of investigational devices is also a critical aspect of our service capabilities, ensuring compliance with local regulations and facilitating smooth project management throughout the trial lifecycle.
Crafting an Effective Clinical Summary Report: Best Practices
To create an effective summary document, it is crucial to follow several best practices that improve clarity and understanding. First and foremost, clarity and conciseness are vital; using straightforward language and minimizing jargon ensures that the document remains accessible to a diverse audience. As Marius Henriksen aptly notes, clarity in the clinical summary report is paramount for effective communication.
A structured format is equally important, as it provides a consistent framework that allows readers to navigate the document effortlessly. For instance, the conclusion section at the end of the document is often presented in bulleted format for ease of the reader, further enhancing clarity. The use of tables and figures to present data visually significantly enhances comprehension and retention, making complex information more digestible.
Furthermore, it is crucial to conduct thorough reviews, ideally through multiple rounds, to eliminate errors and ensure the accuracy of the content. Involving key stakeholders throughout the writing process is also advised, as their insights can enhance the document and tackle any potential biases or unmeasured confounders. This collaborative method can result in enhanced clarity and rigor in the clinical summary report.
For example, observational studies often face limitations in establishing causation; as noted in a case study, conclusions must consider potential biases and unmeasured confounders, as significant associations may arise from true differences, biases, or a combination of both.
By following these practices, researchers can produce high-quality clinical summary reports that effectively communicate their findings, ultimately leading to more impactful contributions to medical research. Recent literature, such as the analysis by Dahlberg et al. on statistical versus practical significance in thoracic malignancies, also emphasizes the importance of these best practices in ensuring that findings are not only statistically significant but practically relevant.
Additionally, incorporating statistical methods like Kaplan-Meier curves and appropriately estimating median follow-up time can further enhance the reporting of survival data.
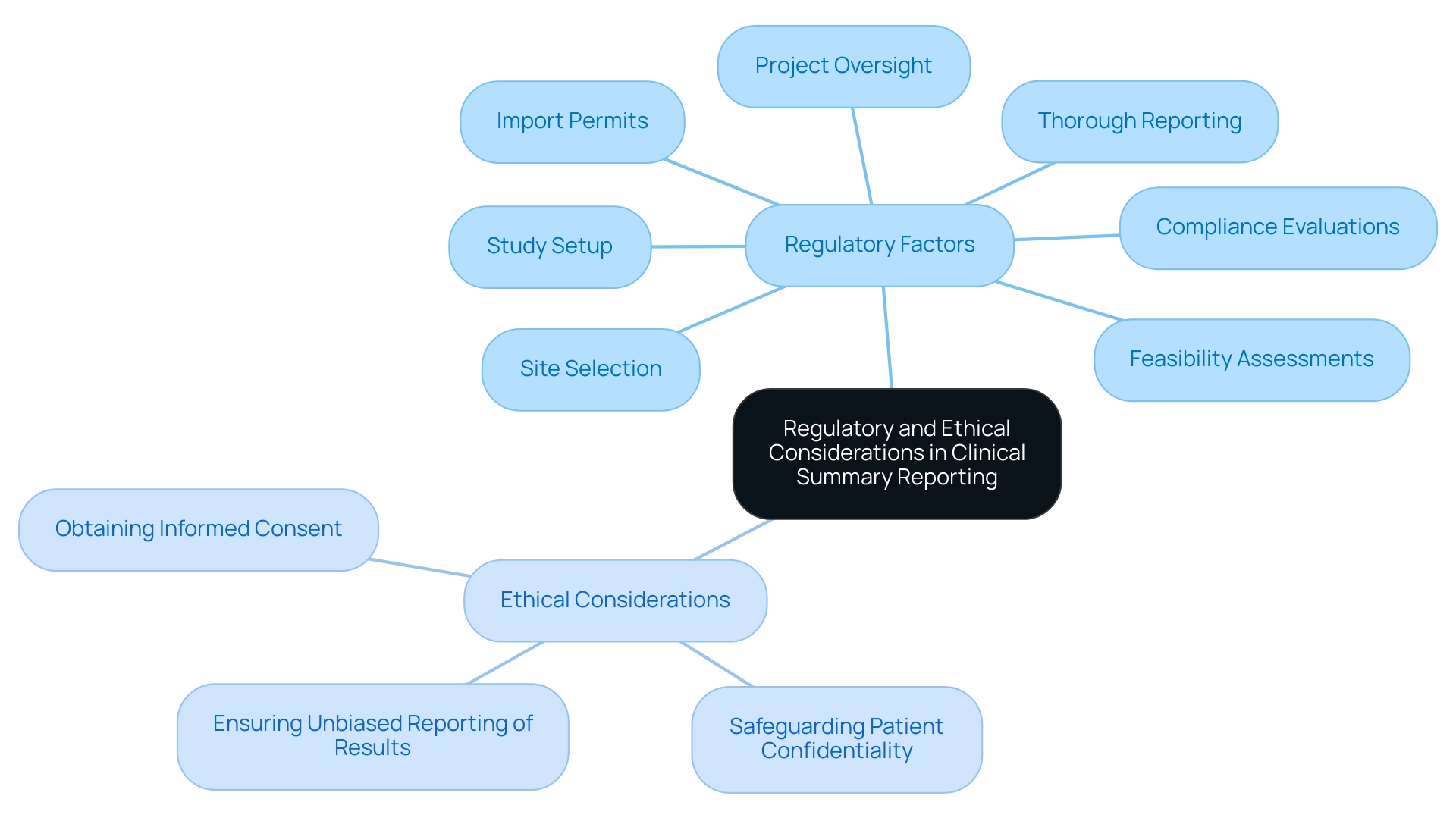
Regulatory and Ethical Considerations in Clinical Summary Reporting
Regulatory and ethical factors are essential in the preparation of clinical summary reports related to trials. Researchers are required to follow stringent guidelines established by regulatory authorities, such as the FDA and EMA, which outline the essential content and format needed for clinical summary reports. Our extensive study management services encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project oversight
- Thorough reporting
Ethical considerations encompass a range of practices, including:
- Safeguarding patient confidentiality
- Obtaining informed consent
- Ensuring unbiased reporting of results
Adhering to these regulations not only protects the rights of participants but also bolsters the credibility of the research findings. As highlighted by Carrol Gamble, PhD, a statistical analysis plan (SAP) encompasses a more technical and thorough explanation of the main aspects of the analysis outlined in the protocol, and includes comprehensive procedures for conducting the statistical analysis of the primary and secondary variables and additional information.
Recent guidance highlights the significance of preserving information from participants who exit studies, further reinforcing the need for strong information integrity and retention policies. With challenges such as regulatory hurdles, competition, recruitment issues, and financial constraints, it is imperative for medical device startups to prioritize compliance with evolving standards. Furthermore, upcoming updates of guidance will take into account changes in legislation and new initiatives related to research studies, ensuring that researchers stay compliant.
The last update to this information was on December 16, 2024, highlighting the importance of staying current with regulatory requirements. A relevant case study, titled 'Data Quality Standards in Clinical Trials,' demonstrates how the FDA's guidance emphasizes the importance of retaining data from subjects who withdraw from clinical trials, ultimately strengthening data integrity and retention policies in clinical research. By prioritizing these regulatory and ethical considerations, researchers can uphold the integrity of their studies and foster trust among all stakeholders involved.
BOOK A MEETING
Conclusion
Clinical summary reports are integral to the successful communication of clinical trial findings, particularly in the rapidly evolving landscape of medical technology in Latin America. These reports provide a comprehensive overview of trial objectives, methodologies, and outcomes, ensuring that all stakeholders—from regulatory bodies to sponsors—are informed and aligned. The detailed structure of these reports, including components such as the executive summary, methods, and results, enhances clarity and facilitates informed decision-making, which is crucial for advancing medical knowledge.
The importance of adhering to best practices in crafting these reports cannot be overstated. Clarity, conciseness, and a structured format are essential for making complex data accessible to diverse audiences. Engaging stakeholders throughout the reporting process further enriches the content and strengthens the integrity of the findings. As the clinical trial landscape continues to evolve, particularly with anticipated changes in reporting standards, the role of clinical summary reports in fostering transparency and collaboration will only become more critical.
Ultimately, by prioritizing the preparation of high-quality clinical summary reports that meet regulatory and ethical standards, researchers can significantly enhance the impact of their studies. These reports not only serve to uphold the credibility of clinical research but also support the broader movement towards increased access to clinical trial data, thereby benefiting patient care and future research initiatives. The ongoing commitment to transparency and rigorous reporting will be vital in navigating the challenges and opportunities that lie ahead in the field of clinical trials.
Frequently Asked Questions
What is a clinical summary report?
A clinical summary report is a crucial document that summarizes the results of a trial, providing an overview of its objectives, methodology, findings, and conclusions. It is essential for stakeholders such as researchers, regulatory bodies, and sponsors to understand research outcomes, especially in the context of challenges faced by Medtech companies in Latin America.
Why are clinical summary reports important for Medtech companies in Latin America?
Clinical summary reports are important for Medtech companies in Latin America because they address challenges like language barriers and scattered resources, ensuring effective communication and collaboration among stakeholders.
How does Bioaccess® contribute to the creation of clinical summary reports?
Bioaccess®, as a leading CRO in Latin America, facilitates the process of creating clinical summary reports by ensuring that study results are accurate and actionable, streamlining regulatory approval processes, and enhancing subject recruitment strategies.
What are the key components of a comprehensive clinical summary report?
The key components of a clinical summary report include: Title Page, Executive Summary, Introduction, Methods, Results, Discussion, Conclusion, and References.
How does the clinical summary report improve transparency and accountability in research?
The clinical summary report enhances transparency and accountability by promoting thorough reporting standards, which builds trust among stakeholders and aligns research objectives with treatment effect descriptions.
What additional features are being incorporated into clinical summary reports?
Increasingly, clinical summary reports are incorporating action buttons that allow users to interact with the data, such as drilling down into summary statistics for specific points, enhancing user engagement and data presentation.
What services does Bioaccess® offer to ensure successful research endeavors?
Bioaccess® offers services such as Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies to ensure that research is compliant and positioned for success in a competitive environment.




