Overview
CROs, or Contract Research Organizations, are essential for medical device trials as they manage the planning, execution, and regulatory compliance of research studies, ensuring efficient collaboration between manufacturers and regulatory bodies. The article emphasizes their importance by detailing the comprehensive services they provide, such as project management and patient recruitment, which facilitate timely and effective research outcomes while navigating complex regulatory landscapes.
Introduction
The role of Contract Research Organizations (CROs) in the medical device industry is increasingly recognized as a cornerstone for successful clinical trials. As specialized entities, CROs provide a comprehensive range of services that streamline the research process, ensuring compliance with stringent regulatory standards while fostering innovation in medical technology.
In regions such as Latin America, where unique challenges abound—from regulatory complexities to communication barriers—CROs like bioaccess® are essential partners that facilitate critical connections between device manufacturers and regulatory bodies.
This article delves into the multifaceted functions of CROs, highlighting their indispensable contributions to clinical trial management, the innovative solutions they implement to navigate regulatory challenges, and the future trends shaping their services in an ever-evolving landscape.
Understanding the pivotal role of CROs is crucial for stakeholders aiming to expedite the development and approval of groundbreaking medical devices, ultimately enhancing patient care and advancing healthcare solutions.
Defining CROs: The Backbone of Medical Device Trials
Contract Research Organizations are specialized entities that offer crucial outsourced research services to the pharmaceutical, biotechnology, and medical equipment sectors. In the field of medical equipment studies, the CRO for Medical Device Trials plays a crucial role in overseeing the planning, execution, and monitoring of research. They effectively bridge the gap between device manufacturers and regulatory bodies, ensuring compliance with rigorous standards and streamlining the research process.
In Latin America, where Medtech companies face unique challenges such as regulatory hurdles and communication barriers, organizations like bioaccess® are crucial. They offer top-notch, budget-friendly services, including:
- Regulatory approval
- Research site activation
- Subject recruitment
- Data management
These services expedite study results and enable smooth cooperation between local hospitals and American research clients.
This is exemplified by the successful first-in-human studies led by companies like PAVmed in Colombia, which showcase the potential for significant advancements in Medtech through strong partnerships. According to Thomas Serena, MD FACS,
SGR has established the standard in research design for chronic wounds, such as diabetic foot ulcers,
reflecting the high benchmarks that top contract research organizations strive to meet. With more than 30 years of expertise, ClinTrial Solutions demonstrates how contract research organizations can support timely and cost-effective research objectives, from protocol inception to regulatory submissions.
Their extensive expertise in CRO for Medical Device Trials enables faster market access for innovative medical devices while ensuring stringent patient safety and data integrity standards are upheld. Moreover, Lindus Health provides an all-encompassing solution addressing every element of research trials, highlighting the varied skills of contract research organizations. The essential function of CRO for Medical Device Trials not only enhances the effectiveness of medical studies but also significantly supports the advancement of healthcare technology, especially in regions like Latin America where closing gaps in research and innovation is vital.
As the field continues to develop, keeping up with the latest advancements in contract research organizations and study management remains vital for research directors.
Key Services Offered by CROs in Medical Device Trials
CRO for Medical Device Trials offer a comprehensive range of services designed to assist medical device studies, including the selection of research locations, principal investigator (PI) identification, and regulatory compliance evaluations. These services encompass:
- Project management
- Study design
- Patient recruitment
- Data management
- Biostatistical analysis
Monitoring services are a critical aspect, ensuring adherence to Good Clinical Practice (GCP) and other regulatory standards.
CROs also provide detailed reporting processes, including regular updates on study status and comprehensive documentation of serious and non-serious adverse events. By managing these multifaceted elements of research—such as Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Post-Market Follow-Up Studies (PMCF)—CRO for Medical Device Trials enables manufacturers to concentrate on their core competencies while ensuring studies are conducted with efficiency and precision. Additionally, the significance of obtaining ethics committee approvals and import permits is paramount in the setup process, ensuring compliance with local regulations.
The CRO market is experiencing significant growth, driven by an increase in new drug approvals and a heightened emphasis on personalized medicine. According to Fortune Business Insights, this growth is attributed to factors like the rising number of new drug approvals and the growing demand for drug safety and efficacy. Recent reports demonstrate that nearly 7,000 medical trials for healthcare products have been registered in the past five years, involving over three million patients, especially concentrating on cardiovascular and oncological conditions.
This robust pipeline emphasizes the crucial role that CRO for Medical Device Trials fulfills in managing the intricacies of clinical studies and regulatory matters, ensuring that all processes comply with the highest standards of safety and efficacy. To learn more about how we can assist you, BOOK A MEETING with our team.
The Importance of CROs in Navigating Regulatory Challenges
CRO for Medical Device Trials play a critical role in navigating the intricate regulatory landscape governing medical device studies. Their extensive knowledge of the requirements established by regulatory authorities, such as the FDA and EMA, is essential for ensuring compliance throughout the study process. Our comprehensive service capabilities include:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Detailed reporting on study status and adverse events
By meticulously aligning research protocols with these regulations, CROss significantly reduce the risks associated with non-compliance, which can result in costly delays or outright rejections of applications. Moreover, their knowledge in regulatory matters, illustrated by figures such as Katherine Ruiz, a specialist in Regulatory Affairs for medical products and in vitro diagnostics in Colombia, boosts the credibility of the trial data, enabling more efficient interactions with regulatory authorities. As Robert Califf, Vice Chancellor for Clinical Research at the Duke Translational Medicine Institute, pointed out, challenges such as the time and financial demands of clinical practice, the overall shortage of specialists, and the increasing complexity of regulations highlight the need for robust support systems in clinical research.
CRO for Medical Device Trials not only mitigate these challenges by providing structured project management and compliance assurance but also bolster the likelihood of successful market approval for medical devices, paving the way for innovations that improve patient care. Additionally, the EMA supports features like concurrent controls and square root allocation in study designs, which contract research organizations incorporate to align with regulatory expectations. The insights from Julio G. Martinez-Clark further emphasize the importance of CROss in Regulatory Affairs and commercialization, showcasing their adaptability in managing the long duration and complexity of research studies.
Innovations and Future Trends in CRO Services for Medical Devices
The landscape of CRO for Medical Device Trials is undergoing significant transformation, driven by rapid technological advancements and evolving regulatory frameworks. Innovations such as artificial intelligence (AI) are playing a crucial role in data analysis, improving the precision and speed of medical studies. Moreover, the incorporation of remote monitoring solutions and the increase of decentralized research studies are transforming conventional study methodologies, encouraging greater patient involvement and enabling more comprehensive data collection processes.
Our thorough research management services include:
- Feasibility studies
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project oversight
- Detailed reporting on study status, inventory, and both serious and non-serious adverse events, ensuring adherence to country requirements.
In Latin America, bioaccess® provides accelerated medical instrument research study services, utilizing over 20 years of expertise to manage:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Research Follow-Up Studies (PMCF) effectively.
The global medical equipment CRO market is anticipated to reach USD 13.3 billion by 2024, with trial management expected to lead, holding around 71% of the market share by 2035.
Recent market dynamics, such as Precision for Medicine's acquisition of Baseline Controls, highlight the competitive landscape within CRO services. Companies like Medpace exemplify this trend; with a focus on organic growth rather than acquisitions, Medpace has reported a remarkable 29.2% increase in revenue, totaling $1.89 billion in 2023. The organization plans to add 1,500 jobs and invest $150 million in expansion, showcasing its commitment to innovation and responsiveness to market demands.
Additionally, Ergomed's strategy of expanding its teams by hiring from top CRO companies while maintaining a small company feel illustrates the importance of talent acquisition in this evolving industry. As noted by Heidi Hennigan, Senior Principal Biostatistician at a top global pharma, Proclinical is currently collaborating with a CRO to recruit for a Senior Principal Biostatistician position, reflecting the current hiring trends in the sector. As the healthcare technology sector keeps progressing, CRO for Medical Device Trials must adopt these innovations to preserve their significance and efficiency as collaborators in medical research.
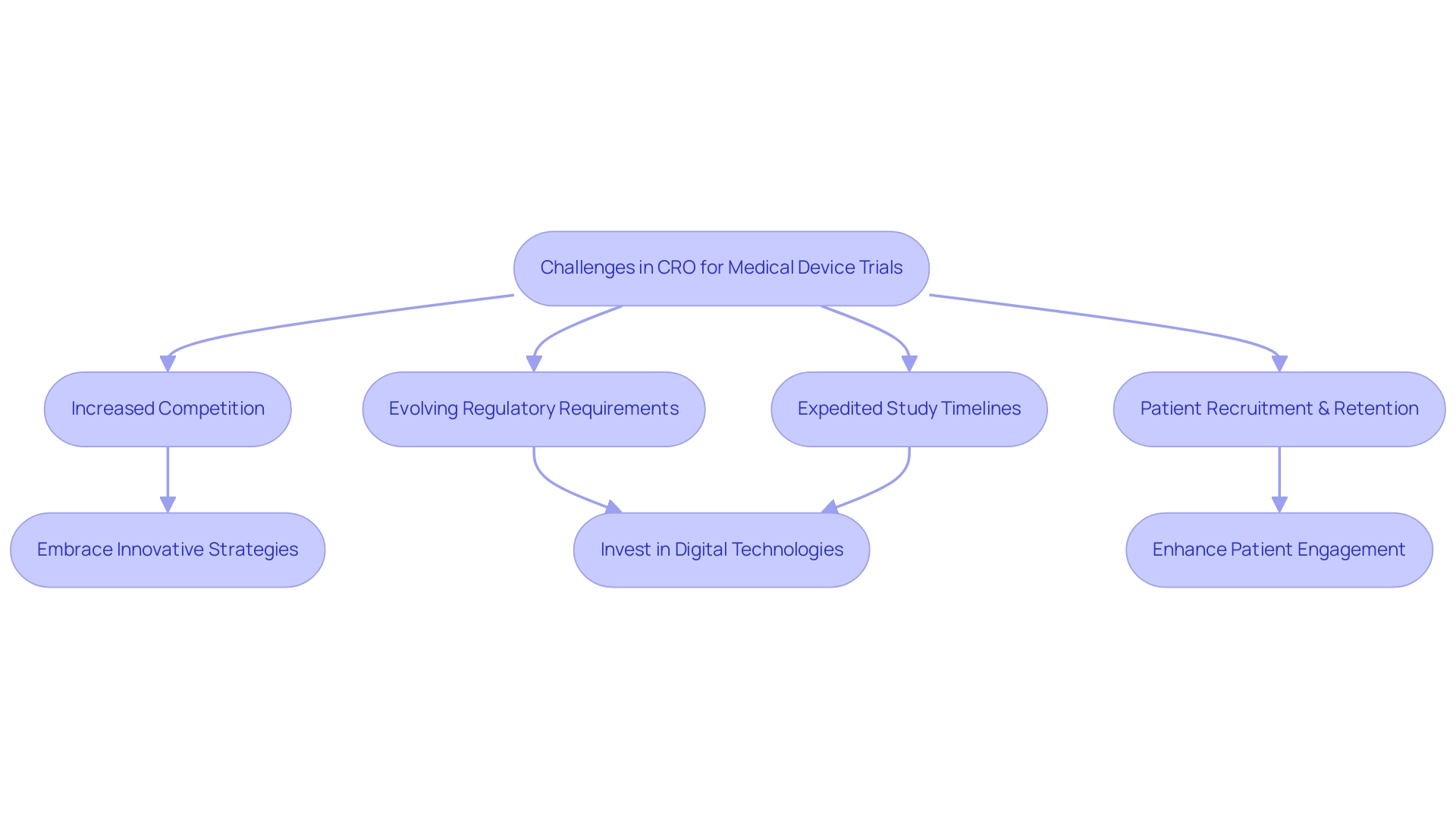
Challenges and Solutions in the CRO Medical Device Industry
Organizations in the medical device sector encounter a multitude of challenges that can hinder their effectiveness in implementing CRO for Medical Device Trials. With North America controlling between 43% and 52% of the overall CRO market, increased competition is a significant concern, alongside evolving regulatory requirements that necessitate rapid adaptation. The need for expedited study timelines further compounds these challenges, making efficient patient recruitment and retention critical for CRO for Medical Device Trials, especially in specialized populations.
Investing in digital technologies offers a considerable chance for clinical research organizations to improve collaboration and simplify clinical study processes. In response to these hurdles, many CROs for medical device trials are embracing innovative strategies. Andrew MacGarvey, CEO of Phastar, observes that increasingly, our customers and potential clients are approaching us with innovative requests, such as incorporating synthetic data into their upcoming studies.
This reflects a broader trend in which technology plays a pivotal role in enhancing patient engagement, particularly in the context of CRO for Medical Device Trials. By utilizing digital tools and encouraging closer cooperation with research sites, CRO for Medical Device Trials are better positioned to improve patient retention rates in trials. Moreover, with over 20 years of expertise in Medtech, bioaccess® is leading the way in providing accelerated medical equipment clinical study services in Latin America.
They focus on various study types including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
Bioaccess® effectively addresses the challenges faced by CRO for Medical Device Trials in the medical sector by adapting to competition and regulatory changes through innovative strategies and digital technologies. The CRO market is expected to grow due to the increasing number of new drug approvals, demand for personalized medicine, and a focus on drug safety and efficacy.
Leading companies in the CRO market, such as Iqvia Holdings Inc., Charles River Laboratories International, and Pra Health Sciences, are leveraging their resources to enhance research methods and expand their market outreach. Investing in staff training ensures that teams remain informed about regulatory changes, enabling them to navigate the complexities of trials effectively. Through these proactive measures, the CRO for Medical Device Trials can maintain high-quality services and facilitate the successful advancement of medical devices within a challenging landscape.
Conclusion
The critical role of Contract Research Organizations (CROs) in the medical device industry cannot be overstated. As specialized entities, CROs provide essential services that streamline the clinical trial process, ensuring compliance with regulatory standards while fostering innovation. This article has explored the multifaceted functions of CROs, from managing clinical studies to navigating complex regulatory landscapes, particularly in regions like Latin America where unique challenges exist.
The comprehensive suite of services offered by CROs—including site selection, patient recruitment, and regulatory compliance—enables medical device manufacturers to focus on their core competencies. By leveraging their expertise, CROs facilitate timely and efficient clinical trials, which are essential for bringing innovative medical technologies to market. Furthermore, the integration of technology and innovative strategies is shaping the future of CRO services, enhancing patient engagement and improving data collection processes.
As the medical device industry continues to evolve, the importance of CROs will only grow. Their ability to adapt to regulatory changes and market demands positions them as indispensable partners for stakeholders looking to expedite the development and approval of groundbreaking medical devices. By understanding and leveraging the capabilities of CROs, stakeholders can significantly enhance patient care and advance healthcare solutions, paving the way for a more innovative and efficient medical technology landscape.
Frequently Asked Questions
What are Contract Research Organizations (CROs)?
Contract Research Organizations (CROs) are specialized entities that provide outsourced research services to the pharmaceutical, biotechnology, and medical equipment sectors.
What role do CROs play in medical device trials?
CROs for Medical Device Trials oversee the planning, execution, and monitoring of research, bridging the gap between device manufacturers and regulatory bodies to ensure compliance with standards and streamline the research process.
What challenges do Medtech companies face in Latin America?
Medtech companies in Latin America face unique challenges such as regulatory hurdles and communication barriers.
What services do organizations like bioaccess® provide to Medtech companies?
Organizations like bioaccess® offer services including regulatory approval, research site activation, subject recruitment, and data management, which help expedite study results and facilitate cooperation between local hospitals and American research clients.
Can you provide an example of successful studies conducted in Latin America?
Successful first-in-human studies led by companies like PAVmed in Colombia exemplify significant advancements in Medtech through strong partnerships.
What expertise do CROs bring to medical device trials?
CROs bring expertise in project management, study design, patient recruitment, data management, and biostatistical analysis, ensuring adherence to Good Clinical Practice (GCP) and regulatory standards.
Why is monitoring services important in CROs?
Monitoring services are critical as they ensure adherence to regulatory standards and Good Clinical Practice (GCP), providing detailed reporting processes on study status and documentation of adverse events.
How does the CRO market trend in recent years?
The CRO market is experiencing significant growth due to an increase in new drug approvals and a rising demand for personalized medicine, with nearly 7,000 medical trials registered in the past five years.
What types of studies do CROs manage?
CROs manage various types of studies including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Post-Market Follow-Up Studies (PMCF).
Why is obtaining ethics committee approvals important in research?
Obtaining ethics committee approvals is essential to ensure compliance with local regulations during the setup process of clinical trials.

