Overview
Global contract research organizations (CROs) play a crucial role in clinical trials by providing comprehensive services that enhance the efficiency and compliance of medical research studies. The article highlights how CROs, such as bioaccess®, facilitate the entire research process—from regulatory approval to patient recruitment—while addressing industry challenges and driving market growth, which is projected to reach USD 93.23 billion by 2032.
Introduction
In the dynamic landscape of clinical research, Global Contract Research Organizations (CROs) have emerged as pivotal players, driving innovation and efficiency in the execution of clinical trials. These specialized firms not only bridge the gap between pharmaceutical companies and regulatory bodies but also offer a comprehensive suite of services tailored to address the unique challenges faced by Medtech startups, particularly in regions like Latin America.
With the global CRO market projected to reach over USD 93 billion by 2032, the increasing reliance on these organizations underscores their vital role in enhancing trial quality and compliance.
As the industry grapples with rising costs, stringent regulations, and the need for swift drug development, the strategic partnerships and innovative practices adopted by CROs are set to redefine the future of clinical research, ensuring that advancements in healthcare are both efficient and equitable.
Defining Global Contract Research Organizations (CROs)
Global contract research organizations (CROs) serve as essential partners in medical research, offering a comprehensive array of services that facilitate the planning, execution, and oversight of research studies. Acting as intermediaries between sponsors—including pharmaceutical companies and biotechnology firms—and regulatory bodies, these specialized firms play a critical role in ensuring compliance and operational efficiency. Among these, bioaccess® stands out as a leading US-based CRO facilitating research specifically for Medtech startups in Latin America.
With a dedication to offering rapid, economical, and top-notch health information, bioaccess® aids clients in maneuvering through the intricacies of regulatory approval, site activation, subject recruitment, and study management. By addressing the regulatory hurdles and resource fragmentation that Medtech companies face in the region, bioaccess® ensures a smoother path to market. According to recent insights from Akash Anand, the global contract research organization market in healthcare was valued at USD 50.38 billion in 2023 and is projected to grow to USD 93.23 billion by 2032, demonstrating a robust compound annual growth rate (CAGR) of 7.10% from 2024 to 2032.
This growth highlights the rising dependence on global contract research organizations to improve study efficiency through their extensive knowledge in design, site management, data collection, and regulatory compliance. Moreover, the partnership between entities such as Greenlight Guru and bioaccess® seeks to expedite Medtech advancements and evaluations in Latin America, tackling the difficulties presented by language barriers, resource fragmentation, and regulatory obstacles. Significantly, the Walmart Healthcare Research Institute, established in October 2022, demonstrates how global contract research organizations can enhance fairness in studies for chronic condition treatments, concentrating on delivering higher-quality and more equitable healthcare through improved research practices.
By streamlining processes and optimizing resource allocation, bioaccess® is proving essential in connecting the gaps in research and innovation, ultimately driving improvements in study quality and results.
Comprehensive Services Provided by CROs in Clinical Trials
Global contract research organizations (CROs) offer a diverse range of services that encompass all stages of clinical studies, including:
- Feasibility assessments
- Investigator selection
- Regulatory compliance
- Project management
- Comprehensive reporting
These offerings ensure a holistic approach to execution, crucial for addressing challenges faced by medical device startups such as:
- Regulatory hurdles
- Competition
- Recruitment issues
- Financial constraints
A key aspect of their services is information management, essential for maintaining the integrity of collected information.
Leading global contract research organizations, such as bioaccess™, employ cutting-edge technologies and extensive data management systems to optimize data gathering, cleaning, validation, and storage, facilitating prompt analysis and informed decision-making that improves overall study efficiency. Specific services also include:
- Review and feedback on study documents to comply with country requirements
- Assistance with import permits and nationalization of investigational devices
Through strategic partnerships, such as bioaccess™ and Caribbean Health Group's initiative to position Barranquilla as a leading location for clinical studies in Latin America, supported by Colombia's Minister of Health, global contract research organizations are paving the way for increased clinical research opportunities.
Moreover, collaborations such as that of GlobalCare Clinical Trials with bioaccess™ have attained over a 50% decrease in recruitment time and 95% retention rates, further showcasing the efficiency of global contract research organizations in enhancing study results. By providing these comprehensive services, global contract research organizations enable sponsors to focus on their core strengths while ensuring that every element of the study is managed effectively, ultimately speeding up research timelines and lowering expenses.
BOOK A MEETING.
The Growing Demand for CROs: Trends and Drivers
The demand for contract research entities has experienced significant growth, driven by several compelling trends. Significantly, increasing research study expenses have driven pharmaceutical firms to pursue more effective operational methods. In 2024, the oncology segment notably dominated the CRO market, underscoring the urgency for rapid drug development timelines amidst complex regulatory landscapes.
As a result, companies are increasingly utilizing the specialized knowledge of global contract research organizations, which provide extensive clinical study management services including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project oversight
- Thorough reporting on study status, inventory, and adverse incidents
Brian Moore, VP of NICCA USA, Inc., stated, 'The quality of research they have done for us has been excellent,' reflecting the high standards contract research organizations maintain. Leading companies in the healthcare CRO market, such as ICON Plc, Charles River Laboratories, Syneos Health, and IQVIA Inc., are at the forefront of this trend, providing essential support to pharmaceutical firms.
Moreover, the strategic collaboration between IQVIA and Argenx, announced in October 2023, aims to advance treatment for rare autoimmune diseases using innovative pharmacovigilance services, enhancing patient safety and treatment efficacy through integrated technology solutions. Furthermore, North America is expected to lead the global CRO market during the forecast period from 2025 to 2034, with recent acquisitions, such as Laboratory Corporation of America Holdings completing its acquisition of Toxikon Corporation, further strengthening the non-clinical development portfolio. The shift towards complex study designs—such as adaptive studies that require flexibility and the inclusion of diverse patient populations—further necessitates the involvement of global contract research organizations.
These organizations, as global contract research organizations, are distinctly situated to manage the obstacles posed by these changing needs, ultimately improving the effectiveness and efficiency of studies while ensuring adherence to national regulations.
Challenges in Clinical Trials: Navigating Regulatory and Recruitment Hurdles
Contract Research Organizations encounter significant challenges in clinical trials, especially concerning regulatory compliance, patient recruitment, and information security. The landscape in 2024 sees regulatory bodies imposing increasingly stringent guidelines, necessitating that global contract research organizations remain vigilant and well-informed about evolving regulations. Non-compliance can lead to significant setbacks, making it imperative for CROs to develop robust strategies for adherence.
In fact, 87% of drug developers are utilizing Full-Service Provider (FSP) or hybrid FSP/Full-Service Organization (FSO) arrangements for their clinical development outsourcing, highlighting the trend towards outsourcing as a means to manage these complexities. Moreover, patient recruitment continues to be a formidable obstacle; the costs associated with finding eligible participants are rising. Recent studies reveal that patient recruitment expenses can account for up to 30% of the total study budget.
To combat these challenges, CROs are adopting innovative recruitment strategies, including:
- Leveraging digital platforms
- Engaging in community outreach
These methods not only improve enrollment rates but also guarantee that studies proceed on schedule. Furthermore, bioaccess® highlights the significance of information security during medical device clinical trials, employing reasonable security measures to safeguard client information while acknowledging the inherent risks involved.
While we strive to ensure the security of the information you transmit, we cannot guarantee absolute security, and any information shared is at your own risk. Effective communication and collaboration between sponsors, global contract research organizations, and third-party vendors are essential for successful information management oversight. As illustrated in a case study, challenges arise from unclear expectations and poor communication channels, but strategies such as:
- Establishing clear communication lines
- Fostering a collaborative environment
can help overcome these hurdles.
Additionally, adopting a modern data approach can enhance data quality and accuracy, streamline report generation, and improve decision-making for Chief Revenue Officers. As Debra Anderson, SVP of Strategic Partnerships, reflects on her journey from a Statistical Programmer to a leader, she underscores the importance of building collaborative teams capable of overcoming these hurdles. The capacity to maneuver through these intricacies is crucial for the prompt and effective implementation of research studies, highlighting the important role organizations like bioaccess® have in progressing medical research.
For any queries or concerns regarding the processing of your information, please contact our Grievance Officer at IMH ASSETS CORP (doing business as 'bioaccess®'), 1200 Brickell Avenue, Suite 1950 #1034, email: info@bioaccessla.com. Additionally, you can manage your cookie preferences at any time by adjusting your browser settings or using the cookie consent banner.
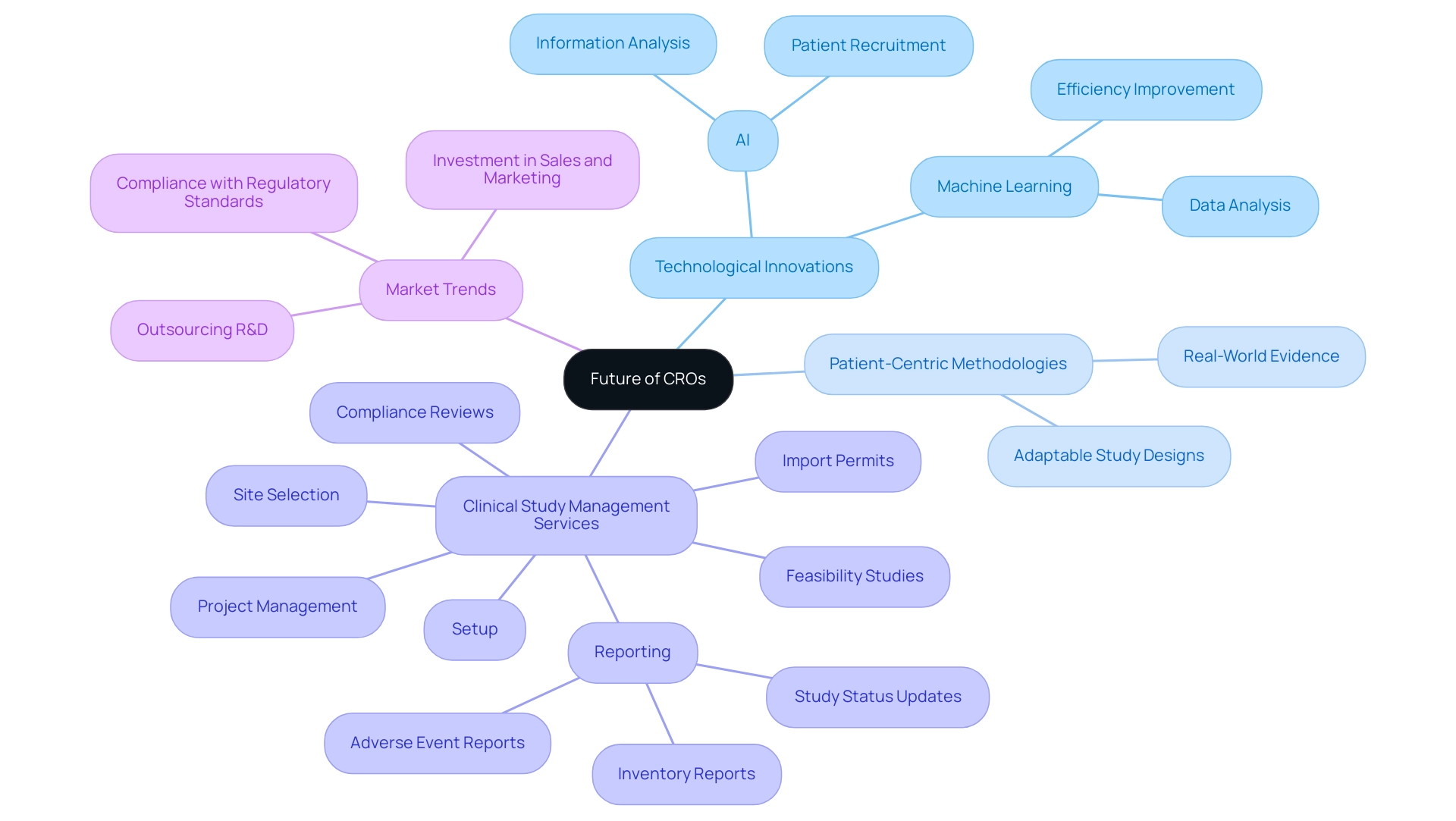
The Future of CROs: Innovations and Evolving Practices
The future of global contract research organizations (CROs) is set for significant transformation, primarily fueled by technological advancements and a pronounced shift towards patient-centric methodologies. Innovations such as artificial intelligence (AI) and machine learning are becoming essential to various phases of clinical studies, especially in areas like information analysis and patient recruitment, which significantly enhance both efficiency and precision. For example, AI can quickly analyze extensive health information collections to identify suitable patients, thereby decreasing recruitment timelines by up to 30%, simplifying study initiation and enhancing overall study efficiency.
As Andrew MacGarvey, CEO of Phastar, observes, 'More and more, our customers and prospective clients are reaching out to us with innovative requests, such as integrating synthetic data into their future studies.' This emphasizes the increasing expectation for clinical research organizations to adapt and innovate. Furthermore, the growing emphasis on patient involvement and the inclusion of real-world evidence are encouraging CROs to adopt more adaptable study designs that prioritize patient needs.
Comprehensive clinical study management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
are critical in this evolving landscape. Notably, reporting encompasses various types of documents, such as study status updates, inventory reports, and adverse event reports, which are essential for maintaining transparency and regulatory compliance throughout the trial process. A notable trend is the increasing reliance of leading pharmaceutical companies on global contract research organizations for efficient drug development, as evidenced by the case study titled 'Increasing Drugs in the Pipeline and Rising Investments in R&D.'
This case study demonstrates how partnerships and investments are improving R&D efficiencies, with many companies outsourcing R&D to global contract research organizations to lower expenses and meet strict drug development timelines. Moreover, the impact of Medtech clinical studies on local economies—through job creation, economic growth, and healthcare improvement—underscores the importance of international collaboration in advancing global health. In this evolving landscape, it is crucial for global contract research organizations to remain agile and responsive, ensuring they deliver the highest quality support to sponsors and play a vital role in advancing medical research.
Additionally, the necessity for CROs to adopt a customer-centric commercial model is paramount; by investing in sales and marketing and being attuned to client needs, CROs can capitalize on emerging opportunities and strengthen their market position. Compliance with country requirements during trial setup is also essential, as it ensures that all regulatory standards are met, thereby facilitating smoother approval processes.
Conclusion
The role of Global Contract Research Organizations (CROs) in clinical research has never been more critical. As detailed throughout the article, CROs like bioaccess® are instrumental in streamlining the complexities of clinical trials, particularly for Medtech startups in regions such as Latin America. By offering a comprehensive suite of services—from regulatory compliance to patient recruitment—CROs enhance trial quality and operational efficiency, which is essential in a rapidly evolving healthcare landscape.
The growth of the CRO market, projected to reach over USD 93 billion by 2032, reflects the increasing demand for their expertise in navigating the multifaceted challenges of clinical trials. As pharmaceutical companies face rising costs and stringent regulations, the strategic partnerships and innovative practices adopted by CROs are pivotal in redefining the future of drug development. The emphasis on patient-centric methodologies and the integration of cutting-edge technologies, such as artificial intelligence, further underscore the transformative potential of CROs in enhancing trial efficiency and accuracy.
In conclusion, as the healthcare industry continues to evolve, the indispensable role of CROs will only grow. By addressing the unique challenges posed by regulatory frameworks and recruitment hurdles, these organizations not only facilitate smoother pathways to market but also drive advancements in healthcare equity and innovation. The collaboration between CROs and Medtech companies promises to yield significant benefits, ensuring that the future of clinical research is both efficient and equitable for all stakeholders involved.
Frequently Asked Questions
What are global contract research organizations (CROs)?
Global contract research organizations (CROs) are specialized firms that provide essential services for medical research, acting as intermediaries between sponsors, such as pharmaceutical companies and biotechnology firms, and regulatory bodies. They help facilitate the planning, execution, and oversight of research studies.
What services do CROs offer?
CROs offer a diverse range of services that encompass all stages of clinical studies, including feasibility assessments, investigator selection, regulatory compliance, project management, and comprehensive reporting.
How does bioaccess® differentiate itself among CROs?
Bioaccess® is a leading US-based CRO that specifically facilitates research for Medtech startups in Latin America, focusing on providing rapid, economical, and high-quality health information while addressing the regulatory hurdles and resource fragmentation these companies face.
What is the projected growth of the CRO market in healthcare?
The global contract research organization market in healthcare was valued at USD 50.38 billion in 2023 and is projected to grow to USD 93.23 billion by 2032, with a compound annual growth rate (CAGR) of 7.10% from 2024 to 2032.
What challenges do medical device startups face that CROs help address?
Medical device startups face challenges such as regulatory hurdles, competition, recruitment issues, and financial constraints. CROs provide support in overcoming these challenges through their comprehensive services.
How do CROs enhance study efficiency?
CROs enhance study efficiency by employing cutting-edge technologies and extensive data management systems to optimize data gathering, cleaning, validation, and storage, facilitating prompt analysis and informed decision-making.
What specific assistance can CROs provide regarding regulatory compliance?
CROs can assist with reviewing and providing feedback on study documents to ensure compliance with country requirements and help with import permits and the nationalization of investigational devices.
How do partnerships between CROs and other organizations benefit clinical research?
Partnerships, such as that between bioaccess® and Caribbean Health Group, aim to increase clinical research opportunities in regions like Latin America. Collaborations have also shown significant improvements in recruitment times and retention rates.
What impact do CROs have on research timelines and costs?
By providing comprehensive services, CROs enable sponsors to focus on their core strengths, effectively managing every element of the study, which ultimately speeds up research timelines and lowers expenses.

