Introduction
The landscape of medical device development is intricately tied to the regulatory framework that governs it, particularly through Investigational Device Exemptions (IDEs). These exemptions are crucial for enabling the use of unapproved devices in clinical trials, allowing researchers to gather essential data on safety and effectiveness that paves the way for market approval. As the FDA emphasizes the importance of compliance and oversight, understanding the nuances of the IDE application process becomes paramount for researchers and medical device innovators alike.
This article delves into the key aspects of IDEs, including:
- Their definition
- The criteria for exemption
- The application process
- The FDA's role in approval
- The ongoing responsibilities researchers must uphold post-approval
All of which are vital for advancing medical technology while ensuring patient safety and regulatory adherence.
Defining Investigational Device Exemptions (IDE)
To understand what is an IDE study, it is important to note that an Investigational Device Exemption (IDE) allows the use of instruments not yet approved by the FDA in clinical trials aimed at collecting essential safety and effectiveness data. This oversight route is essential for advancing medical instruments through the research stage, as it helps researchers understand what is an IDE study to collect the required data for market authorization. IDEs not only facilitate the evaluation of innovative medical technologies but also ensure adherence to standards, as outlined in 21 CFR 820 Subpart C, which mandates design controls within the Quality System Regulation.
By offering a systematic method for clinical trials, what is an IDE study greatly increases the chances of successful approval, ultimately aiding in better patient outcomes. Furthermore, the comprehensive clinical trial management services offered by bioaccess® address critical aspects such as:
- Site feasibility
- Investigator selection
- Trial setup
- Import permits
- Regulatory compliance
This mitigates challenges faced by medical startups including recruitment issues and financial constraints. The FDA underscores the importance of engaging with investigators early in the process, stating, "Additionally, FDA accepts Pre-Submissions, in which you can submit information to FDA and receive advice on topics such as a non-clinical testing plan and/or a draft clinical protocol."
Additionally, IDEs have played a crucial role in influencing Medicare coverage for certain research, especially via the guidelines set forth under the Medicare Prescription Drug, Improvement, and Modernization Act of 2003. This framework has fostered a more centralized review process for IDE coverage, establishing:
- Coverage A for routine care without equipment coverage
- Coverage B for both routine care and equipment coverage, contingent on meeting specific criteria
It is also important to note that posters related to these studies are due by March 11, 2024, at 11:59 p.m. EST, emphasizing the importance of timely submissions in the research process.
To learn more about how we can assist with your clinical trials, BOOK A MEETING today.
IDE Exemption Criteria and Risk Assessment
To qualify for an Investigational Device Exemption (IDE), an instrument must fulfill specific criteria, particularly regarding its potential to pose significant risks to patients. The FDA categorizes instruments into three primary groups:
- Class I, which generally necessitates minimal regulatory oversight;
- Class II, which entails moderate regulations;
- Class III, where items encounter the most rigorous requirements due to their higher risk profile.
Central to what is an IDE study is the application process, which involves a comprehensive risk assessment that evaluates the potential hazards associated with its use in clinical studies.
This assessment is vital in ensuring that the anticipated benefits of the product substantially outweigh its risks, thereby prioritizing patient safety throughout the research process. Furthermore, the cover letter for an IDE application must include:
- Equipment information
- Sponsor details
- Any applicable waiver requests
These elements are crucial for a complete application. An approved investigational apparatus exemption allows a product to be shipped lawfully for investigations, underscoring the significance of this regulatory pathway.
As noted by Frank Samuelson from the FDA, understanding the implications of artificial intelligence and machine learning in developing in-vivo diagnostic tools presents unique challenges that must be navigated in conjunction with these assessments. Additionally, our service capabilities encompass:
- Feasibility and selection of research locations and principal investigators
- Compliance reviews of project documents
- Trial setup and approval processes
- Import permits and nationalization of investigational devices
- Comprehensive project management and reporting
A pertinent case example is the Emergency Use Deviations, where in urgent situations, deviations from the investigational plan to protect subjects do not require FDA approval but must be reported within 5 working days after the sponsor learns of the deviation.
This provision ensures that patient safety is prioritized while maintaining oversight. The oversight environment is changing, especially with the newest standards for what is an IDE study and exemptions in 2024, which must be thoughtfully evaluated in the context of risk management and the effects of Medtech clinical trials on local economies, including job creation and healthcare enhancement. Katherine Ruiz, a specialist in Regulatory Affairs for medical products and in vitro diagnostics in Colombia, highlights the significance of comprehending these changing regulations to maneuver through the intricacies of clinical trials efficiently.
Navigating the IDE Application Process
Understanding what is an IDE study involves a multi-faceted application process that begins with the meticulous preparation of the application itself. This document must encompass comprehensive details about the medical device, what is an IDE study, and the research protocol. A crucial element of this process is obtaining approval from an Institutional Review Board (IRB), which is essential for ensuring ethical oversight and protection of participants, especially when considering what is an IDE study.
To enhance the application’s integrity, it is important to provide additional supportive information and justifications for any omissions. Once submitted to the FDA, a standard review period of approximately 30 days begins, during which the agency assesses compliance with legal standards. Clear communication and thorough documentation are paramount throughout this period to facilitate a smooth approval process.
Furthermore, contract research organizations (CROs) such as bioaccess® can assist in planning what is an IDE study to ensure compliance with standards while considering scientific validity and ethical implications. This includes crucial services such as trial setup, start-up, and obtaining necessary approvals from ethics committees and health ministries, as well as securing import permits and nationalizing investigational devices. This collaborative effort between researchers and regulatory bodies is vital for understanding what is an IDE study and ensuring the success of the IDE application.
For instance, the recent research comparing GAE using Embosphere microspheres versus corticosteroid injections for knee osteoarthritis exemplifies what is an IDE study, as it has received CMS approval on October 20, 2023. Such examples emphasize the significance of comprehensive clinical trial management services, including feasibility assessments, site selection, compliance reviews, and strong project management, especially in the dynamic environment of medical device research in Latin America.
The FDA's Role in IDE Review and Approval
The FDA plays a crucial role in the review and approval of Investigational Device Exemption (IDE) applications, ensuring a thorough evaluation of several critical elements. The agency scrutinizes the scientific validity of the proposed research, assesses the qualifications of the research team, and examines the adequacy of the informed consent process. Furthermore, the review process encompasses a detailed analysis of potential risks to participants, ensuring that robust measures are in place to mitigate those risks.
This thorough supervision is essential for preserving patient safety and guaranteeing compliance with regulations during the research. As Andrew Potter from the FDA/CDER emphasizes,
How to determine a clinically important difference in free-living measures,
the FDA's role extends beyond approval; it is central to fostering a research environment that prioritizes patient safety. Recent advancements, such as the CMS approval on 2023-10-20 of the research titled 'GAE Using Embosphere Microspheres vs Corticosteroid Injections,' which compares Embosphere microspheres with corticosteroid injections for knee osteoarthritis treatment, further emphasize the FDA's impact on advancing investigational equipment trials and promoting safe, effective therapies.
In this framework, bioaccess® offers expedited clinical research services for medical technology in Latin America, utilizing over 20 years of expertise to oversee early-feasibility assessments, first-in-human trials, and pivotal evaluations, ensuring adherence to compliance requirements. Our services include trial setup, site selection, compliance reviews, and project management, all tailored to facilitate the FDA review process effectively. Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, plays a key role in guiding these efforts.
This integrated approach not only enhances the quality of clinical research but also contributes positively to local economies through job creation and international collaboration.
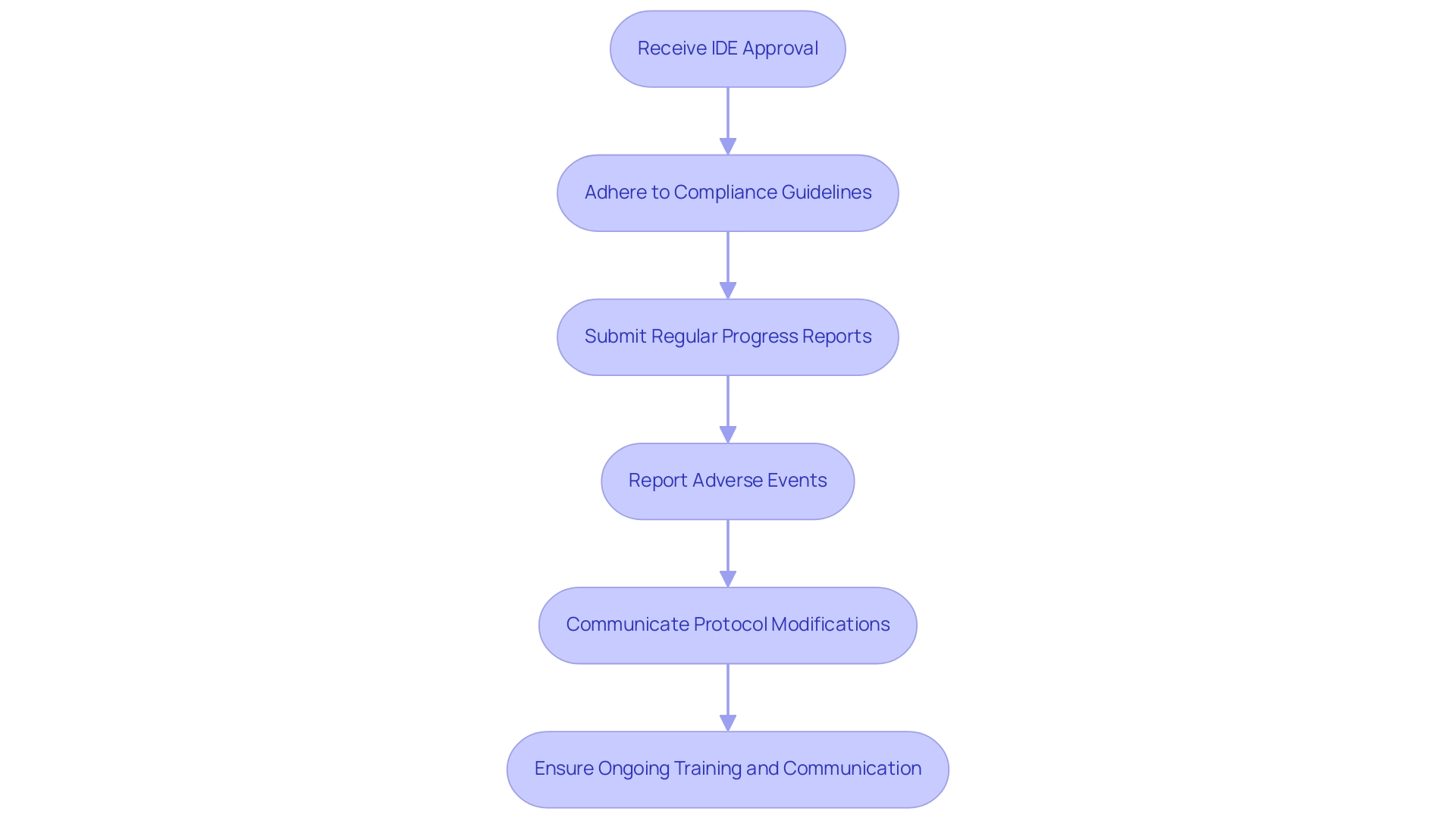
Maintaining Compliance: Responsibilities After IDE Approval
Upon receiving IDE approval, researchers are tasked with adhering to stringent compliance guidelines throughout the duration of the research. This includes the submission of regular progress reports to the FDA and the prompt reporting of any adverse events encountered during the research, both serious and non-serious. Precise record-keeping is essential to accurately represent the research's conduct.
Additionally, researchers must ensure that any modifications to the protocol are communicated effectively to both the FDA and the Institutional Review Board (IRB). Our service capabilities facilitate this process by providing comprehensive clinical trial management services, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Nationalization of investigational devices
- Project management
- Reporting
Recent research, such as the GAE analysis sponsored by Merit Medical Systems, Inc., which received CMS approval on October 20, 2023, underscores the emphasis on compliance.
Ongoing training and transparent communication within the research team are essential for maintaining compliance and protecting the integrity of the project. This diligence ultimately contributes to the advancement of medical knowledge and enhances patient outcomes. As noted by the FDA, 'Additionally, FDA accepts Pre-Submissions, in which you can submit information to FDA and receive advice on topics such as a non-clinical testing plan and/or a draft clinical protocol.'
This quote highlights the proactive approach researchers can take in ensuring compliance responsibilities after IDE approval. Furthermore, the case study on Cardiac Resynchronization Therapy, which compared His/Left Bundle Branch Pacing to Biventricular Pacing and was also approved by CMS on October 20, 2023, serves as a pertinent example of compliance in clinical research, illustrating the importance of thorough reporting of adverse events. The commitment to compliance not only aligns with regulatory expectations but also reinforces the ethical standards inherent in clinical research, supported by experts like Katherine Ruiz in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia.
Conclusion
The Investigational Device Exemption (IDE) is a pivotal regulatory pathway that facilitates the use of unapproved medical devices in clinical trials, enabling researchers to collect essential safety and effectiveness data. Understanding the criteria for exemption, the intricacies of the application process, and the ongoing responsibilities post-approval is crucial for advancing medical technology while ensuring patient safety. The FDA's comprehensive oversight throughout this process underscores the importance of regulatory compliance in fostering an environment conducive to innovation.
The application process for an IDE is multi-dimensional, involving:
- Detailed preparations
- Ethical oversight from Institutional Review Boards
- Thorough risk assessments
These elements are essential for ensuring that the benefits of a device substantially outweigh its risks. Furthermore, the role of the FDA in reviewing applications and maintaining rigorous standards of safety and efficacy cannot be overstated. Recent developments, such as the approval of studies comparing innovative treatment methods, highlight the dynamic nature of medical device research and the critical importance of regulatory frameworks in supporting these advancements.
Post-approval compliance responsibilities are equally vital, as researchers must maintain rigorous standards in reporting and adherence to study protocols. This commitment to compliance not only aligns with regulatory expectations but also reinforces the ethical standards that underpin clinical research. By fostering effective communication and ongoing training within research teams, the integrity of studies is upheld, ultimately contributing to improved patient outcomes and the successful advancement of medical devices in the market. The collaborative efforts between researchers, regulatory bodies, and clinical trial management services are essential for navigating the complexities of the IDE process and achieving meaningful innovations in healthcare.
Frequently Asked Questions
What is an Investigational Device Exemption (IDE) study?
An IDE study allows the use of medical instruments that are not yet approved by the FDA in clinical trials to collect essential safety and effectiveness data, facilitating the advancement of medical technologies.
Why are IDEs important in clinical trials?
IDs provide a systematic method for conducting clinical trials, increasing the chances of successful approval for medical devices and ultimately improving patient outcomes.
What are the key components of the IDE application process?
The IDE application process includes a comprehensive risk assessment, a cover letter that contains equipment information, sponsor details, and any applicable waiver requests.
How does the FDA classify medical instruments for IDEs?
The FDA classifies instruments into three categories: Class I (minimal regulatory oversight), Class II (moderate regulations), and Class III (most rigorous requirements due to higher risk).
What role do IDEs play in Medicare coverage?
IDs influence Medicare coverage through guidelines established by the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, which delineates Coverage A (routine care without equipment coverage) and Coverage B (routine care and equipment coverage under specific criteria).
What challenges do medical startups face regarding IDEs?
Medical startups often face recruitment issues and financial constraints, which can be mitigated by utilizing comprehensive clinical trial management services.
What are some services offered to assist with clinical trials related to IDEs?
Services include site feasibility, investigator selection, trial setup, import permits, and ensuring regulatory compliance.
What is the significance of timely submissions in the research process for IDE studies?
Timely submissions, such as poster submissions due by March 11, 2024, are crucial for maintaining the research timeline and ensuring compliance with regulatory requirements.
What is an Emergency Use Deviation in the context of IDEs?
An Emergency Use Deviation allows for deviations from the investigational plan in urgent situations to protect subjects without requiring prior FDA approval, although these deviations must be reported within 5 working days.
How is the oversight environment for IDEs changing?
The oversight environment is evolving with new standards for IDE studies and exemptions in 2024, necessitating careful evaluation in the context of risk management and the impact of Medtech clinical trials on local economies.

