Overview
Clinical evaluation is defined as a systematic process that assesses the safety and efficacy of healthcare products and treatments through rigorous data analysis, ensuring compliance with regulatory standards. The article emphasizes that this comprehensive evaluation not only validates performance but also informs stakeholders about real-world outcomes, which are crucial for patient safety and healthcare improvement, thus highlighting its foundational role in the medical device industry.
Introduction
In the realm of healthcare, the significance of clinical evaluation cannot be overstated. This rigorous process not only ensures the safety and effectiveness of medical devices and treatments but also serves as the backbone of regulatory compliance and patient trust.
With an ever-evolving landscape of medical technology, stakeholders must navigate a complex web of clinical data analysis, regulatory requirements, and innovative methodologies.
From early feasibility studies to post-market surveillance, the clinical evaluation process is pivotal in safeguarding patient health and enhancing clinical outcomes.
As advancements in the field continue to emerge, understanding the nuances of clinical evaluation becomes essential for professionals aiming to contribute meaningfully to medical innovation and public health.
Defining Clinical Evaluation: An Overview
Medical assessment acts as a thorough structure aimed at determining both the safety and efficacy of healthcare products and therapies through extensive data analysis. This systematic method, essential for successful medical device trials, involves meticulous collection and evaluation of evidence to ensure that products not only meet regulatory standards but also fulfill their intended purposes. By utilizing the expertise of bioaccess®, which has over 20 years in Medtech, stakeholders can benefit from comprehensive trial management services, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies
Our tailored approach guarantees adaptability in managing the intricacies of trials, which is essential for adjusting to the distinct requirements of each project. Recent research suggests that the MeSH-based approach obtains a wider range of distinct MeSH terms, improving the assessment process. Significantly, 88.2% of domain-specific relevant terms assessed by subject matter experts (SMEs) are included within this framework, which highlights its reliability in collecting essential medical data.
Insights gained from these assessments inform stakeholders about the product's real-world performance, essential for protecting patient health and enhancing health outcomes. As Farkas, R. observes, 'Guidance for Clinical Assessment under the Medical Device Regulation through Automated Scoping Searches' is essential for comprehensive analyses. Maria Nyåkern, Ph.D., emphasizes that strong assessments are foundational for medical device safety, necessitating alignment with EU MDR and ISO standards.
Furthermore, the continuous development of medical data analysis techniques mirrors the necessity for ongoing enhancement in assessment processes. A recent case analysis on the BERT-Based Semantic Search Method highlighted its effectiveness in retrieving domain-specific information while posing challenges in transparency and understandability, illustrating the need for further research to enhance interpretability. Ultimately, these advancements not only impact patient safety outcomes but also foster trust in medical innovations, contributing to economic growth and job creation in local economies.

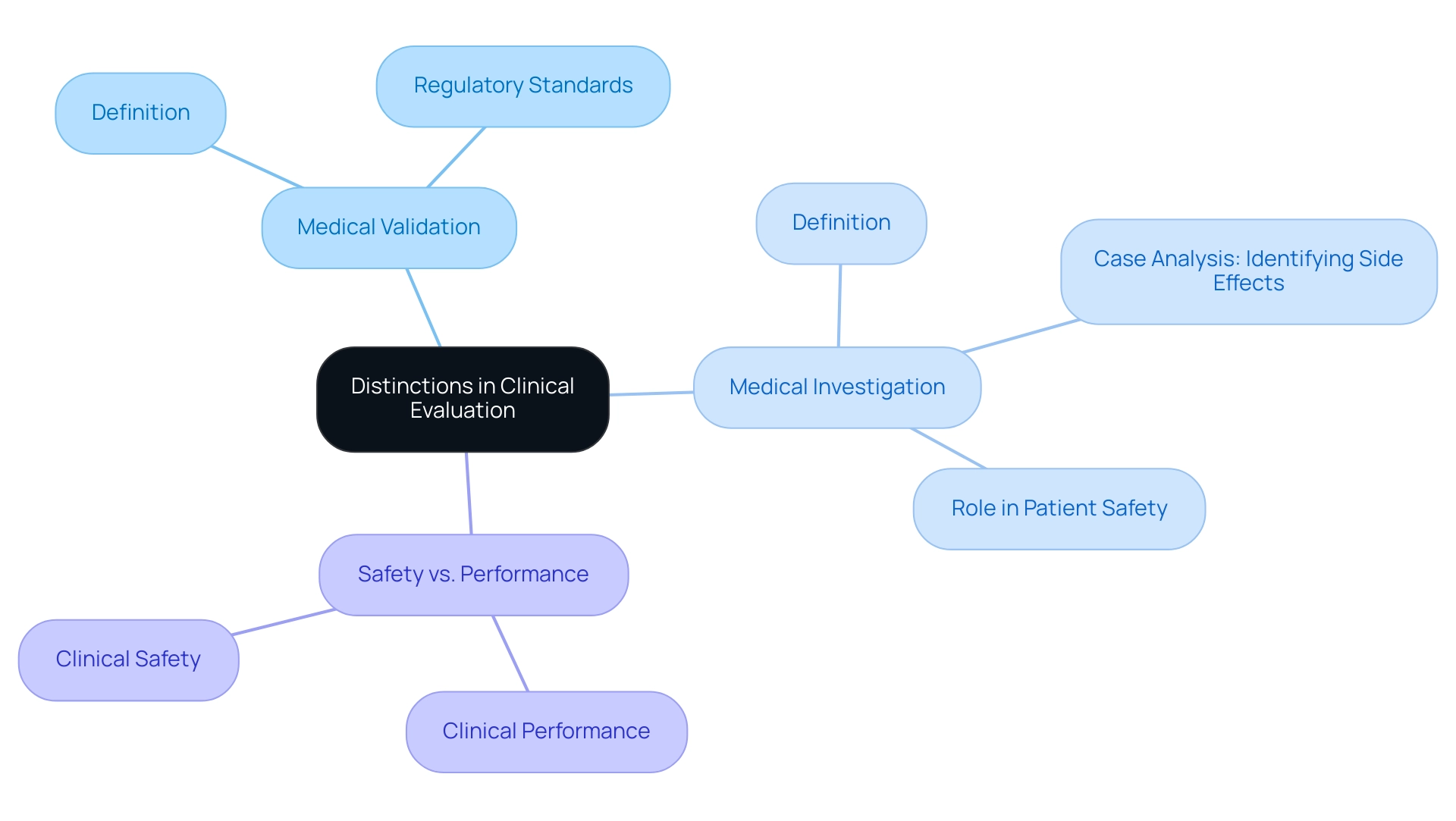
Distinguishing Clinical Evaluation from Related Concepts
Medical assessment is often misunderstood as equivalent to medical validation and medical investigation. To clarify, medical validation involves the process of confirming that a healthcare instrument performs as intended, while investigation refers to the actual study of a tool or treatment within a medical environment. In contrast, the clinical evaluation definition is a comprehensive process that not only validates performance but also entails a thorough review of all pertinent data to ensure adherence to regulatory standards, particularly in the context of diverse regulatory environments like Colombia's INVIMA.
As Maria Nyåkern, Ph.D., a prominent consultant in the medical device industry, observes, she possesses extensive practical experience in establishing and overseeing numerous studies involving tens of thousands of patients across various indications. This extensive experience underscores the necessity for professionals in medical research and development to grasp these distinctions. Moreover, medical investigations play a crucial role in recognizing known side effects and revealing previously unknown ones, as demonstrated by the case analysis titled 'Identifying Side Effects.'
This case analysis emphasizes how medical assessments aid in a better comprehension of the safety characteristics of healthcare tools, which is crucial for improving patient care and guaranteeing that these tools adhere to the highest safety standards. It is essential to note that medical performance should not be confused with medical safety, as the latter encompasses a broader understanding of how devices impact patient health. Through expedited research services offered by bioaccess®, including Early-Feasibility and First-In-Human trials, we provide a complete range of services that encompasses trial setup, project management, compliance reviews, and reporting.
This integration of services not only facilitates successful clinical trials but also supports the economic growth and healthcare improvement in the region, highlighting the significant impact of Medtech clinical studies on local economies.
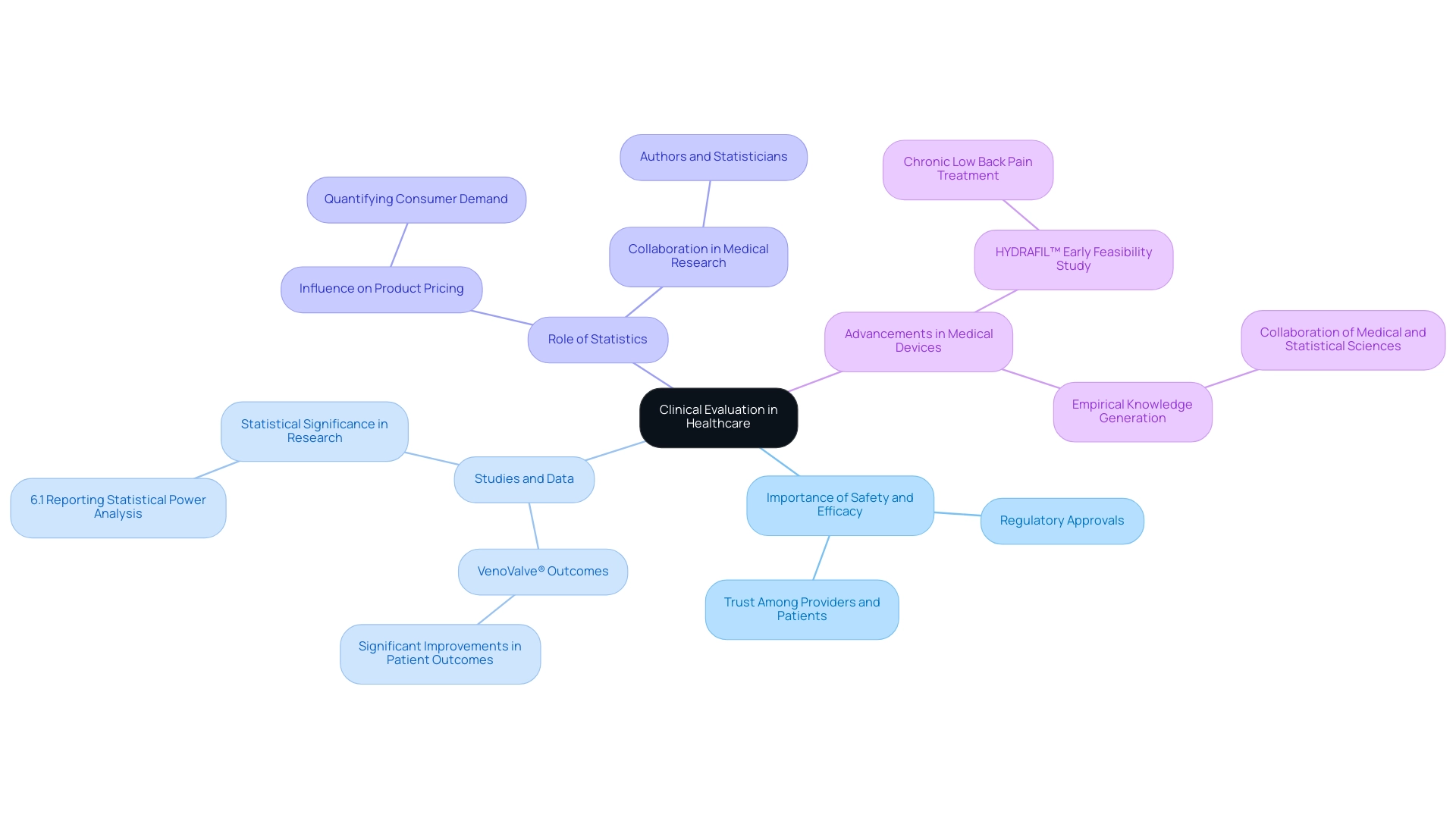
The Importance of Clinical Evaluation in Healthcare
The clinical evaluation definition is fundamental to the healthcare sector, ensuring that medical devices and treatments meet safety and efficacy standards for patient use. These assessments produce the empirical evidence required for regulatory approvals, thereby fostering trust among healthcare providers and patients. Recently, Dr. Jorge Hernando Ulloa presented one-year first-in-human data on the VenoValve® at the prestigious Charing Cross International Symposium, the longest-running vascular and endovascular global symposium in Europe, highlighting advancements in vascular medicine that are essential to assessment processes.
The findings from the VenoValve® research demonstrated significant improvements in patient outcomes, highlighting the device's potential in treating venous insufficiency. A staggering statistic reveals that in a review of 49 studies reporting findings without statistical significance, only three (6.1%) reported a statistical power analysis, highlighting a critical gap that thorough research can address. Larry B. Wallnau emphasizes,
Statistics are equally important to pharmaceutical and technology companies in developing product lines that meet the needs of the populations they serve.
Moreover, the clinical evaluation definition illustrates how statistics play a crucial role in influencing product pricing by quantifying consumer demand, underscoring the importance of thorough evaluations. Additionally, ReGelTec's Early Feasibility Study on HYDRAFIL™ for treating chronic low back pain in Colombia represents another vital advancement in medical practices. The case study titled 'Empirical Knowledge Generation' illustrates how both medical and statistical sciences collaborate to produce empirical knowledge, demonstrating that establishing a hypothesis requires a theoretical basis in biology supported by statistical analysis of observed data.
In an era when patient safety is of supreme significance, the clinical evaluation definition highlights that comprehensive assessments are essential to maintain high standards of medical practice and guarantee effective healthcare delivery.
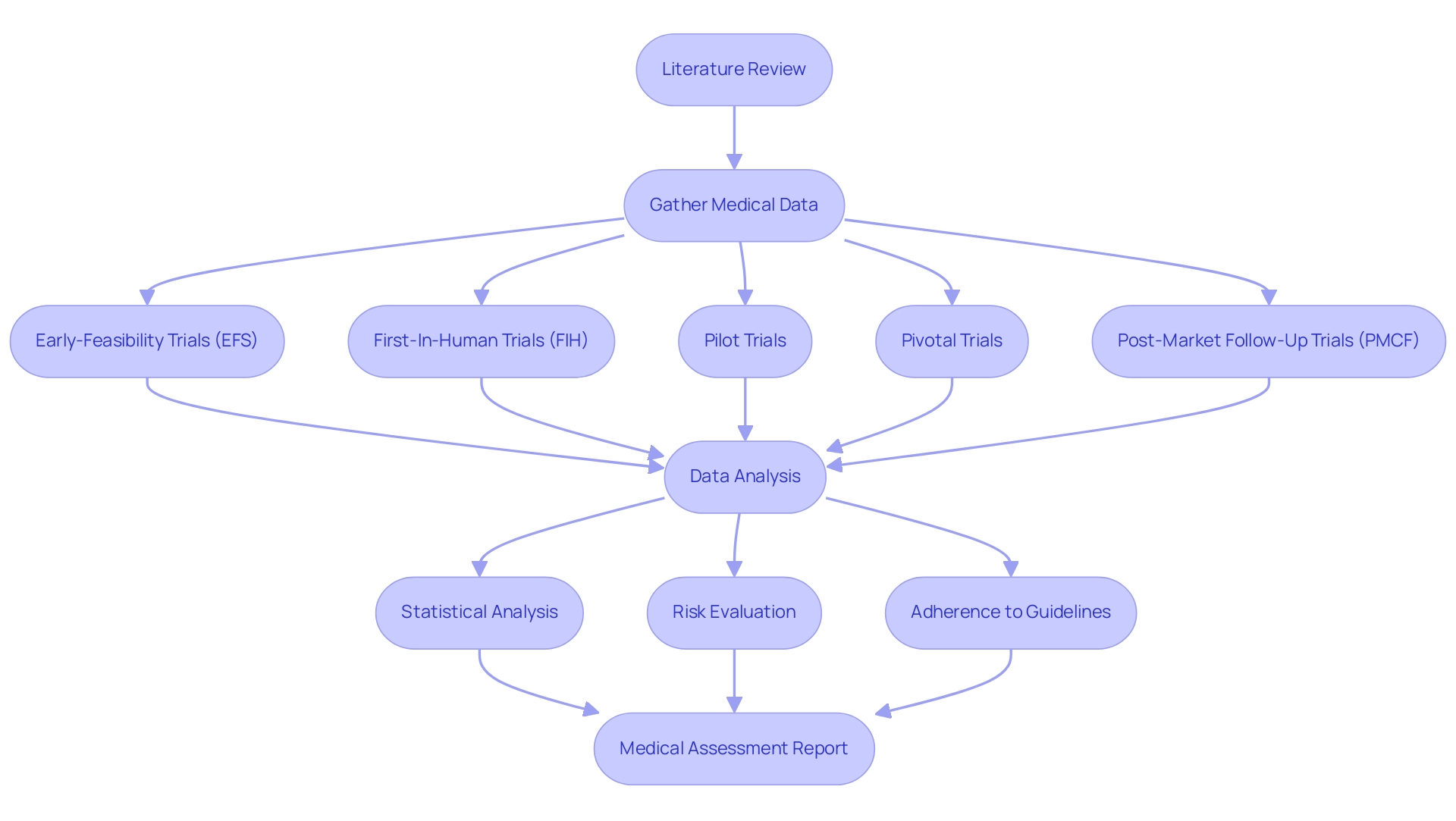
Understanding the Clinical Evaluation Process
The medical assessment process is defined by a methodical and organized approach, beginning with a comprehensive literature review. This critical step aims to gather and synthesize existing data related to the device or treatment in question. Subsequently, medical data is gathered through both trials and observational research, which can take five years or more to finish, highlighting the extensive dedication needed in evaluations.
The analysis of this data focuses on the clinical evaluation definition, which assesses the safety and efficacy of the intervention. Key methodologies used during this phase include:
- Rigorous statistical analysis
- Comprehensive risk evaluation
- Adherence to established medical guidelines
Significantly, bioaccess® utilizes over 20 years of knowledge in Medtech, overseeing various research including:
- Early-Feasibility Trials (EFS)
- First-In-Human Trials (FIH)
- Pilot Trials
- Pivotal Trials
- Post-Market Follow-Up Trials (PMCF)
This ensures the highest standards in trial management.
Our customized approach allows us to tailor services to meet specific client needs, enhancing flexibility throughout the process. Recent advancements in the field have highlighted the value of non-experimental designs, such as epidemiological studies and historically-controlled trials, which can provide compelling evidence of treatment efficacy. The conclusion of this process is the medical assessment report, an essential document that not only aids regulatory submissions but also serves an important function in post-market monitoring.
Furthermore, understanding INVIMA's role as Colombia's National Food and Drug Surveillance Institute is essential for compliance, as it serves as a Level 4 health authority by PAHO/WHO. As noted by Niki Price, a seasoned professional in the medical device industry, her journey began in production, revealing the profound importance and satisfaction derived from this line of work. Ultimately, the effectiveness of the medical assessment process is enhanced by routine pilot trials and careful planning in relation to the clinical evaluation definition, particularly concerning statistical power and sample sizes.
This is additionally backed by the document 'Comments on Statistical Approaches in Clinical Research,' which examines the necessity for strong study designs to tackle methodological weaknesses, thus emphasizing the significance of meticulous planning and execution in the assessment process.
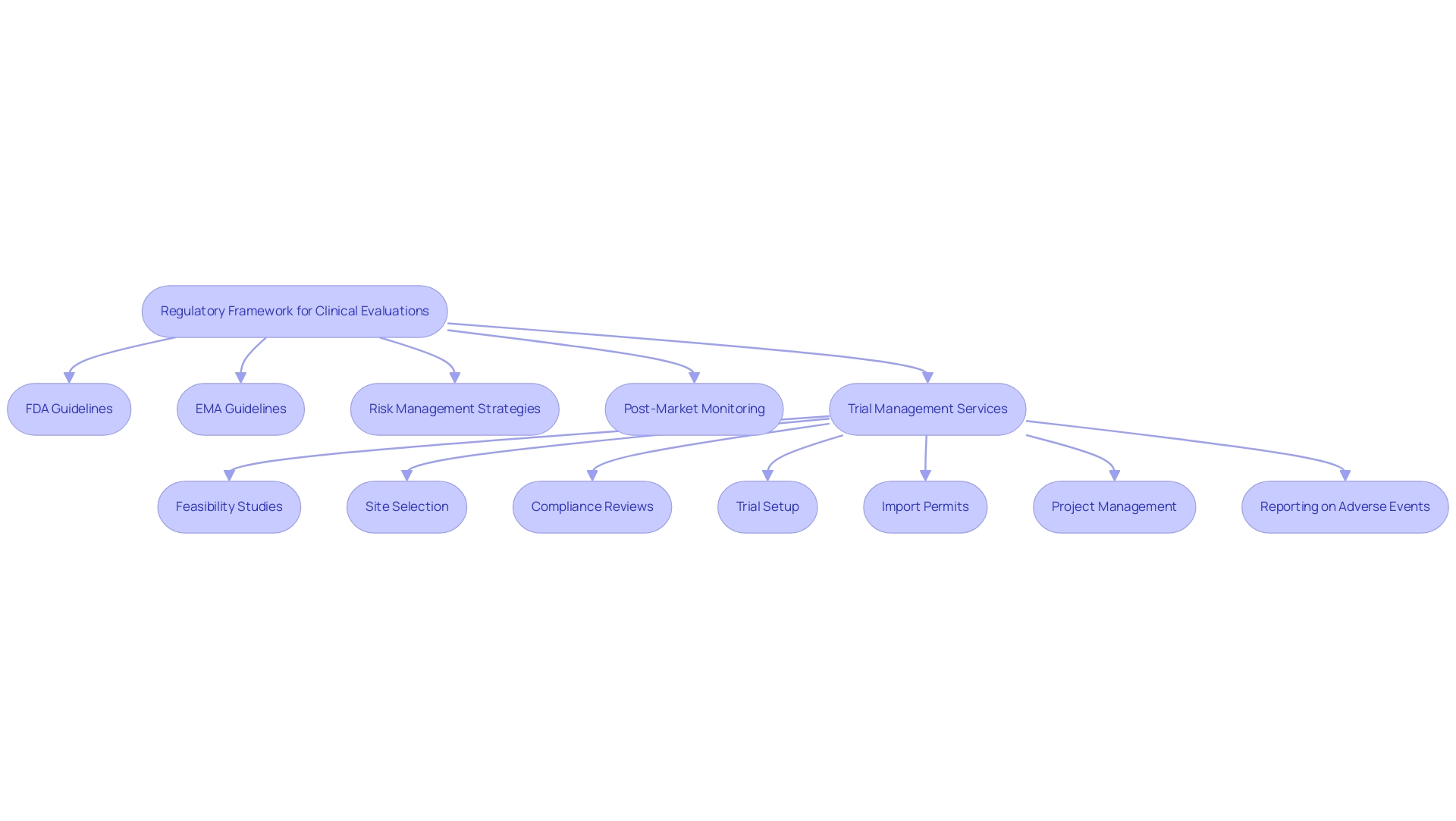
Regulatory Framework and Requirements for Clinical Evaluations
The regulatory environment for medical assessments is mainly influenced by the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe. These agencies establish comprehensive guidelines, such as the ICH E9 document, which provide the clinical evaluation definition and essential requirements for conducting evaluations. The emphasis is on strong medical data, effective risk management strategies, and diligent post-market monitoring.
Adherence to these regulations is paramount, not only for securing market approval but also for safeguarding the ongoing safety and efficacy of medical products. Our comprehensive trial management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup—including ethics committee and health ministry approvals
- Import permits
- Project management
- Detailed reporting on serious and non-serious adverse events
This ensures that researchers have the necessary support to navigate this complex landscape. Experts like Ana Criado, Director of Regulatory Affairs, and Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, provide invaluable insights to enhance compliance and facilitate successful trial outcomes.
Recent updates in regulatory requirements highlight the evolving nature of compliance, underscoring the need for researchers in the healthcare field to stay informed. According to Dr. Carrol Gamble, recommendations are offered for a minimum set of items that should be addressed in Statistical Analysis Plans (SAPs) for research trials. This insight illuminates the importance of meticulous reporting practices, as emphasized by the FDA's reporting principles advocating for clear context and comprehensive details in presenting results.
Furthermore, the agreement between two tests can change significantly based on the proportion of subjects with and without the condition of interest, adding complexity to the assessment process. Understanding these guidelines equips clinical researchers to adeptly navigate the intricate regulatory environment, ensuring the integrity and reliability of their clinical evaluation definition. Notably, the case study titled 'Reporting Recommendations' underscores the FDA's essential reporting principles, reinforcing the need for comprehensive reporting.
Conclusion
Clinical evaluation stands as a cornerstone of modern healthcare, ensuring that medical devices and treatments are both effective and safe for patient use. Through a systematic approach that encompasses literature reviews, clinical trials, and observational studies, stakeholders can gather and analyze critical data that informs regulatory compliance and enhances patient outcomes. The insights derived from these evaluations not only support the approval process but also foster trust among healthcare providers and patients, promoting a culture of safety and efficacy within the medical community.
The distinctions between clinical evaluation, validation, and investigation are crucial for professionals in the field. Understanding these differences enables stakeholders to navigate the complexities of regulatory environments and highlights the importance of thorough data analysis. Moreover, advancements in methodologies, such as the MeSH-based approach and non-experimental designs, showcase the ongoing evolution of clinical evaluation processes, reinforcing the necessity for continuous improvement in the assessment of medical technologies.
In conclusion, as the landscape of healthcare evolves, the significance of robust clinical evaluations cannot be overstated. These evaluations play a vital role in safeguarding patient health, driving medical innovation, and ensuring that healthcare delivery meets the highest standards. By committing to rigorous evaluation processes and adhering to regulatory guidelines, the medical community can uphold its responsibility to provide safe and effective treatments, ultimately contributing to improved health outcomes and fostering public trust in medical advancements.
Frequently Asked Questions
What is the purpose of medical assessment in healthcare?
Medical assessment aims to determine the safety and efficacy of healthcare products and therapies through extensive data analysis, ensuring that products meet regulatory standards and fulfill their intended purposes.
What types of studies are included in the trial management services offered by bioaccess®?
The trial management services include Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies.
How does the MeSH-based approach enhance the assessment process?
The MeSH-based approach improves the assessment process by obtaining a wider range of distinct MeSH terms, with 88.2% of domain-specific relevant terms assessed by subject matter experts included within this framework, enhancing the reliability of medical data collection.
Why are insights from medical assessments important for stakeholders?
Insights from medical assessments inform stakeholders about a product's real-world performance, which is essential for protecting patient health and improving health outcomes.
What is the difference between medical validation, medical investigation, and clinical evaluation?
Medical validation confirms that a healthcare instrument performs as intended, medical investigation studies a tool or treatment in a medical environment, while clinical evaluation is a comprehensive process that validates performance and reviews all pertinent data to ensure adherence to regulatory standards.
How do medical investigations contribute to patient safety?
Medical investigations help identify known side effects and reveal previously unknown ones, enhancing the understanding of healthcare tools' safety characteristics and ensuring adherence to high safety standards.
What services does bioaccess® provide to facilitate clinical trials?
Bioaccess® offers expedited research services, including Early-Feasibility and First-In-Human trials, covering trial setup, project management, compliance reviews, and reporting.
What impact do Medtech clinical studies have on local economies?
Medtech clinical studies support economic growth and healthcare improvement in the region, highlighting their significant impact on local economies.

