Introduction
In the realm of clinical trials, the evolution of Electronic Data Capture (EDC) systems marks a significant shift towards enhanced data management and operational efficiency. These innovative systems are designed to replace traditional paper-based methodologies, offering real-time data entry and monitoring that aligns with stringent regulatory standards.
As the market for EDC technologies is projected to grow substantially in the coming years, understanding their capabilities becomes essential for stakeholders aiming to improve the integrity and accuracy of clinical research. This article delves into the multifaceted benefits of EDC systems, their key features, the challenges faced during implementation, and the future trends that promise to reshape the landscape of clinical trials, ultimately driving better patient outcomes and fostering economic growth in the healthcare sector.
Understanding Electronic Data Capture (EDC) Systems in Clinical Trials
Electronic data capture clinical platforms represent a transformative advancement in the management of information produced during clinical trials. Created to replace conventional paper-based techniques, these solutions for electronic data capture clinical enable a more streamlined and efficient method for gathering information. With the EDC technology market anticipated to witness a compound annual growth rate (CAGR) of 10.6% from 2024 to 2031, the uptake of these innovations is becoming progressively essential.
Electronic data capture clinical frameworks facilitate real-time information entry and observation, a crucial role that guarantees adherence to strict regulatory standards. By offering a centralized platform for thorough information management, including open-source and progressive web app features, these solutions for electronic data capture clinical significantly improve the integrity and precision of clinical research information. Abu Saleh Mohammad Mosa, Director of Research Informatics at the University of Missouri Institute for Clinical and Translational Science, expresses the benefits succinctly:
The use of EDC platforms enhances accuracy of information, reduces research-related expenses, minimizes overall collection time, and ensures integrity, security, and compliance with HIPAA.
Moreover, the incorporation of extensive clinical study management services—such as feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting—enhances the functionality of EDC platforms, ensuring a comprehensive approach to clinical investigations. This includes critical processes such as obtaining ethics committee and health ministry approvals, as well as reviewing and providing feedback on study documents to comply with country requirements. The increase in decentralized clinical studies generates profitable opportunities for market participants, as electronic data capture clinical frameworks facilitate remote information gathering and real-time oversight, making their execution not only a strategic benefit but also an essential element for enhancing decision-making and patient results.
Moreover, Medtech clinical studies play a crucial role in local economies by fostering job creation, stimulating economic growth, and enhancing healthcare outcomes through international collaboration. A relevant case study entitled 'Substantial differences in perception of disease severity between post COVID-19 patients and healthcare professionals' highlights the significance of precise information capture in clinical trials, revealing considerable discrepancies that can influence treatment strategies.
Benefits of Implementing EDC Systems in Clinical Research
The use of electronic data capture clinical frameworks in clinical research significantly improves information accuracy and integrity while optimizing operational efficiency. By transitioning from conventional information gathering techniques to automated processes, EDC solutions effectively minimize manual entry mistakes, thereby lowering the risk of discrepancies. Recent studies indicate that EDC systems can enhance information accuracy, fostering an environment of trust and reliability in research findings.
This is supported by statistical tests conducted at a two-sided significant level of 0.05, validating the enhancements in accuracy. Furthermore, the ability for immediate information access accelerates decision-making, allowing researchers to quickly recognize and tackle possible problems during the experiment. This proactive approach not only accelerates study timelines but also enhances patient safety, ultimately leading to more dependable research outcomes.
For instance, a comparative study revealed that the EDC process decreased data collection costs by 55%, with potential savings ranging from 49% to 62% when contrasted with paper-based data collection methods. According to Maryam Y. Garza, PhD, MPH, MMCi from the University of Arkansas for Medical Sciences, 'All authors reviewed and interpreted the results and read and approved the final version,' underscoring the collective commitment to ensuring high-quality content and outcomes in clinical investigations. Moreover, as part of comprehensive clinical study management services, bioaccess® excels in the feasibility and selection of research sites and principal investigators, compliance reviews, study setup, and project management.
Our compliance with ICJME recommendations highlights the importance of maintaining high standards in research practices. The reporting of study status, inventory, and adverse events is also a critical component of our services, ensuring transparency and accountability throughout the research process. As organizations increasingly adopt electronic data capture clinical solutions, the benefits become evident—not only in financial terms but also in the overall enhancement of research quality and compliance with industry standards, ultimately driving global health improvement and fostering international collaboration in the medtech sector.
Key Features and Functionalities of EDC Systems
The functionality of electronic data capture clinical systems is essential in modern clinical studies, featuring several key aspects that improve usability and compliance. User-friendly interfaces simplify information entry, making the process efficient and intuitive for researchers. Strong validation procedures guarantee the precision and dependability of collected information, while extensive reporting features offer detailed insights for decision-making, essential in the context of comprehensive clinical research management services.
Our service abilities encompass feasibility studies, compliance evaluations, and site selection that enhance the application of electronic data capture clinical technologies, ensuring that studies are organized and carried out effectively. EDC solutions aid in experiment preparation by offering templates and workflows that direct researchers through the process, while their reporting features enable real-time updates on study advancement and information integrity. Many systems for electronic data capture clinical, like Veeva Vault EDC, which has powered over 1,000 study starts, also integrate smoothly with other clinical trial management systems, encouraging seamless information exchange across platforms.
Additional functionalities, including electronic signatures and audit trails, enhance security and compliance with regulatory standards, adhering to privacy laws like HIPAA and GDPR. Real-time monitoring capabilities further enhance data handling, allowing researchers to maintain oversight and adjust strategies as needed. Furthermore, the anticipated compound annual growth rate (CAGR) of 15.8% for EDC technologies from 2024 to 2033 emphasizes the significance of these features in enhancing management of studies and improving patient results, ultimately aiding in job creation, economic development, and healthcare advancement in local economies.
The selection of Oracle’s pharmacovigilance and clinical research solutions by Clinixir Company Limited exemplifies the practical application of electronic data capture clinical tools in enhancing the efficiency of clinical studies, showcasing how these resources support comprehensive project management throughout the study lifecycle.
Challenges in Implementing Electronic Data Capture Systems
The implementation of electronic data capture clinical systems in clinical trials presents several significant challenges. Among these, staff resistance to change stands out, particularly from those familiar with traditional information collection methods. Recent reports indicate that this resistance can hinder the adoption of EDC technologies, creating a barrier to efficient data management.
Notably, Veeva Vault EDC has powered more than 1,000 study starts, showcasing the potential success of EDC implementation when effectively managed. Furthermore, organizations often require extensive training to equip their teams with the necessary skills to navigate new technologies. It is crucial to address skill gaps within cloud computing teams through targeted assessments and collaborative tools, which can facilitate smoother transitions to EDC platforms.
Furthermore, potential integration challenges with current setups may arise, complicating the transition process. Data security and compliance with regulatory requirements also present ongoing challenges that must be meticulously managed. To overcome these hurdles, organizations should prioritize early engagement with stakeholders, fostering an environment of open dialogue to address any concerns and build consensus.
As highlighted by the quote, "Establish effective rapport with your coaching clients by active listening, setting clear boundaries, and maintaining transparency in the coaching relationship," effective communication is essential in overcoming staff resistance.
Comprehensive training programs tailored to specific workflows can further alleviate resistance, ensuring that the chosen electronic data capture clinical solution not only meets regulatory standards but also aligns effectively with organizational needs. The case study titled "Finding Common Ground on Account Plans" illustrates how encouraging open dialogue can effectively address conflicts related to EDC adoption. By proactively tackling these challenges, clinical research teams can enhance their information management processes and facilitate successful electronic data capture clinical adoption.
Future Trends and Innovations in Electronic Data Capture
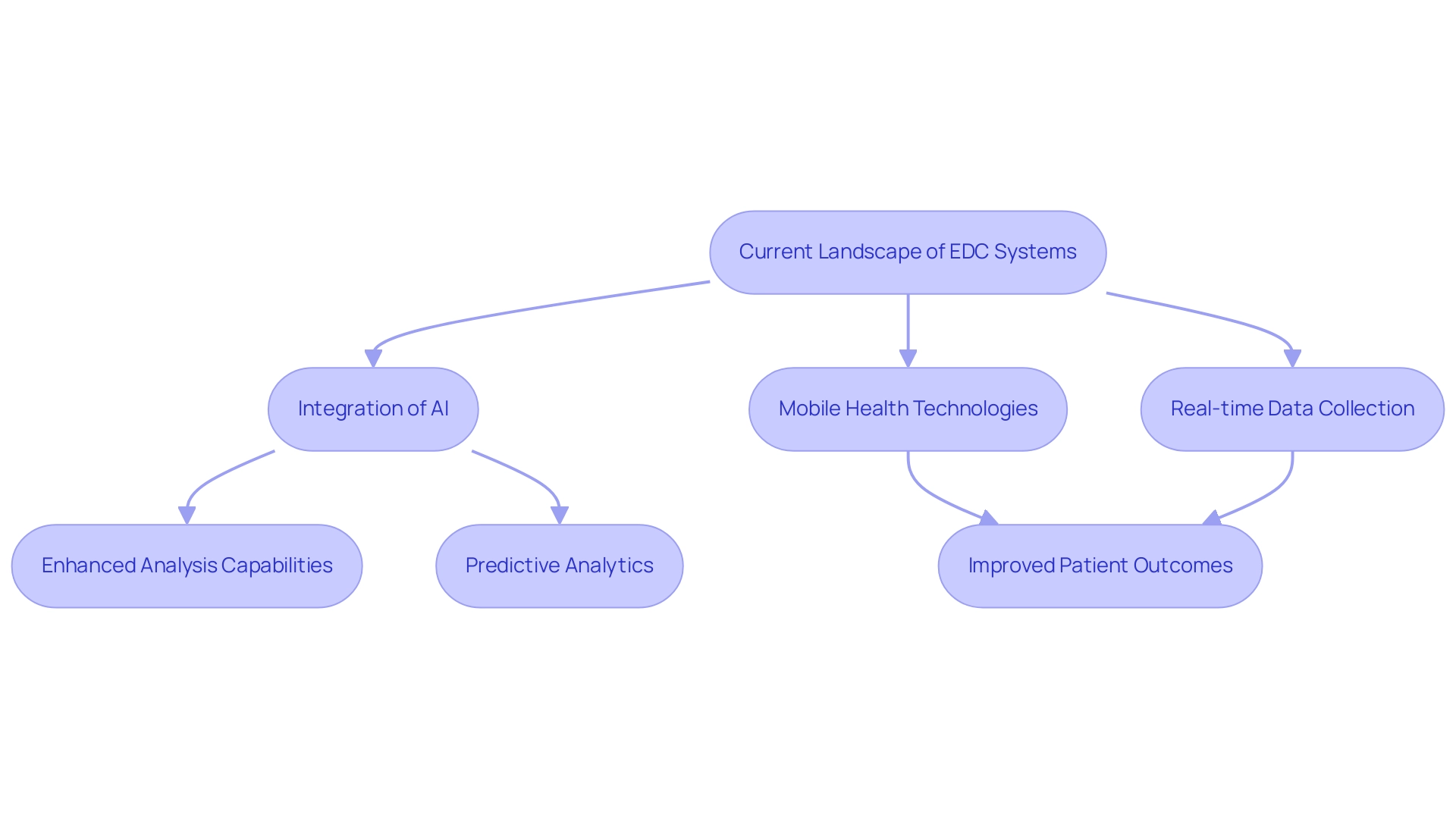
The landscape of electronic data capture clinical systems is on the brink of a major transformation, especially with the integration of artificial intelligence (AI) and mobile health technologies. These advancements are set to enhance analysis capabilities significantly, enabling predictive analytics that can pinpoint trends and potential challenges before they escalate. Furthermore, mobile health applications are facilitating more adaptable information collection methods, empowering researchers to gather real-time insights directly from patients, thereby enhancing engagement and compliance.
As these technologies continue to evolve, the role of electronic data capture clinical solutions in the clinical trial process is anticipated to become increasingly vital, promoting greater efficiency and ultimately improving patient outcomes. As mentioned by business formation specialist Steve Bennett, this article intends to highlight the key trends and statistics of EDC software in 2024, offering stakeholders the insights required to navigate and thrive in the constantly changing realm of information capture. The ongoing growth, with functional service provider outsourcing by large biopharma companies expanding at over 13% annually, further underscores the importance of these innovations.
Additionally, Veeva Systems Inc.'s financial performance, as highlighted in Table 79, reflects the increasing investment in EDC technologies and their critical role in the market. Understanding these shifts, exemplified in the case study on the strategic importance of EDC software, reveals that businesses can develop strategies that leverage technological advancements, ensuring they remain leaders in their respective fields. The recent initiative 'Working with Physicians' also emphasizes the need for improved physician access to patient data, further illustrating the evolving landscape of electronic data capture clinical systems and their implications for clinical research.
Conclusion
The integration of Electronic Data Capture (EDC) systems into clinical trials represents a pivotal advancement in the field of clinical research. By transitioning from traditional paper-based methods to automated data collection, these systems enhance data accuracy and operational efficiency, ultimately leading to improved patient outcomes. The benefits of EDC systems, including:
- Real-time data access
- Streamlined processes
- Cost reductions
are becoming increasingly recognized as essential components for successful clinical trials.
Despite the numerous advantages, the implementation of EDC systems is not without challenges. Resistance to change among staff, the need for extensive training, and potential integration issues with existing systems can hinder the transition to these innovative technologies. However, proactive engagement and comprehensive training can mitigate these obstacles, facilitating smoother adoption and enhancing overall data management processes.
Looking ahead, the future of EDC systems is poised for transformation through the integration of artificial intelligence and mobile health technologies. These innovations promise to further refine data analysis and collection methods, enabling researchers to engage more effectively with patients and make informed decisions in real time. As the market for EDC technologies continues to grow, stakeholders must remain adaptable and informed about these trends to leverage the full potential of EDC systems in driving successful clinical trials and fostering economic growth in the healthcare sector.
Frequently Asked Questions
What are electronic data capture (EDC) clinical platforms?
EDC clinical platforms are advanced solutions designed to replace traditional paper-based methods for managing information produced during clinical trials, enabling a more efficient and streamlined process for data gathering.
What is the expected growth rate for the EDC technology market?
The EDC technology market is anticipated to witness a compound annual growth rate (CAGR) of 10.6% from 2024 to 2031.
How do EDC platforms improve clinical research?
EDC platforms facilitate real-time information entry and observation, enhancing the accuracy, integrity, and compliance of clinical research data while reducing research-related expenses and overall collection time.
What are some key features of EDC systems?
Key features of EDC systems include centralized information management, open-source capabilities, progressive web app functionalities, and extensive clinical study management services such as feasibility assessments and compliance evaluations.
How do EDC platforms support decentralized clinical studies?
EDC platforms enable remote information gathering and real-time oversight, making decentralized clinical studies more feasible and enhancing decision-making and patient outcomes.
What economic impacts do Medtech clinical studies have?
Medtech clinical studies contribute to local economies by creating jobs, stimulating economic growth, and improving healthcare outcomes through international collaboration.
What are the benefits of using EDC systems compared to traditional methods?
EDC systems can reduce data collection costs by 55% and minimize manual entry mistakes, which lowers the risk of discrepancies and enhances the reliability of research findings.
What role does immediate information access play in clinical research?
Immediate information access accelerates decision-making, allowing researchers to quickly identify and address potential issues during experiments, thereby enhancing patient safety and research outcomes.
How does compliance with industry standards relate to EDC systems?
EDC systems help ensure compliance with industry standards, such as ICJME recommendations, which is critical for maintaining high-quality research practices and transparency throughout the research process.

