Introduction
In the intricate realm of healthcare, the performance testing of medical devices stands as a critical pillar in ensuring patient safety and device efficacy. This rigorous evaluation process encompasses a variety of methodologies aimed at assessing the functionality, reliability, and compliance of devices with established clinical standards. As the medical devices market continues to expand, projected to reach a staggering US$233.50 billion by 2029, the demand for comprehensive performance evaluations becomes increasingly paramount.
In this context, organizations must navigate complex regulatory landscapes and adhere to stringent quality assurance practices to foster trust among healthcare providers and patients alike. This article delves into the multifaceted aspects of medical device performance testing, exploring its significance, methodologies, and the regulatory frameworks that underpin this essential process.
Defining Medical Device Performance Testing
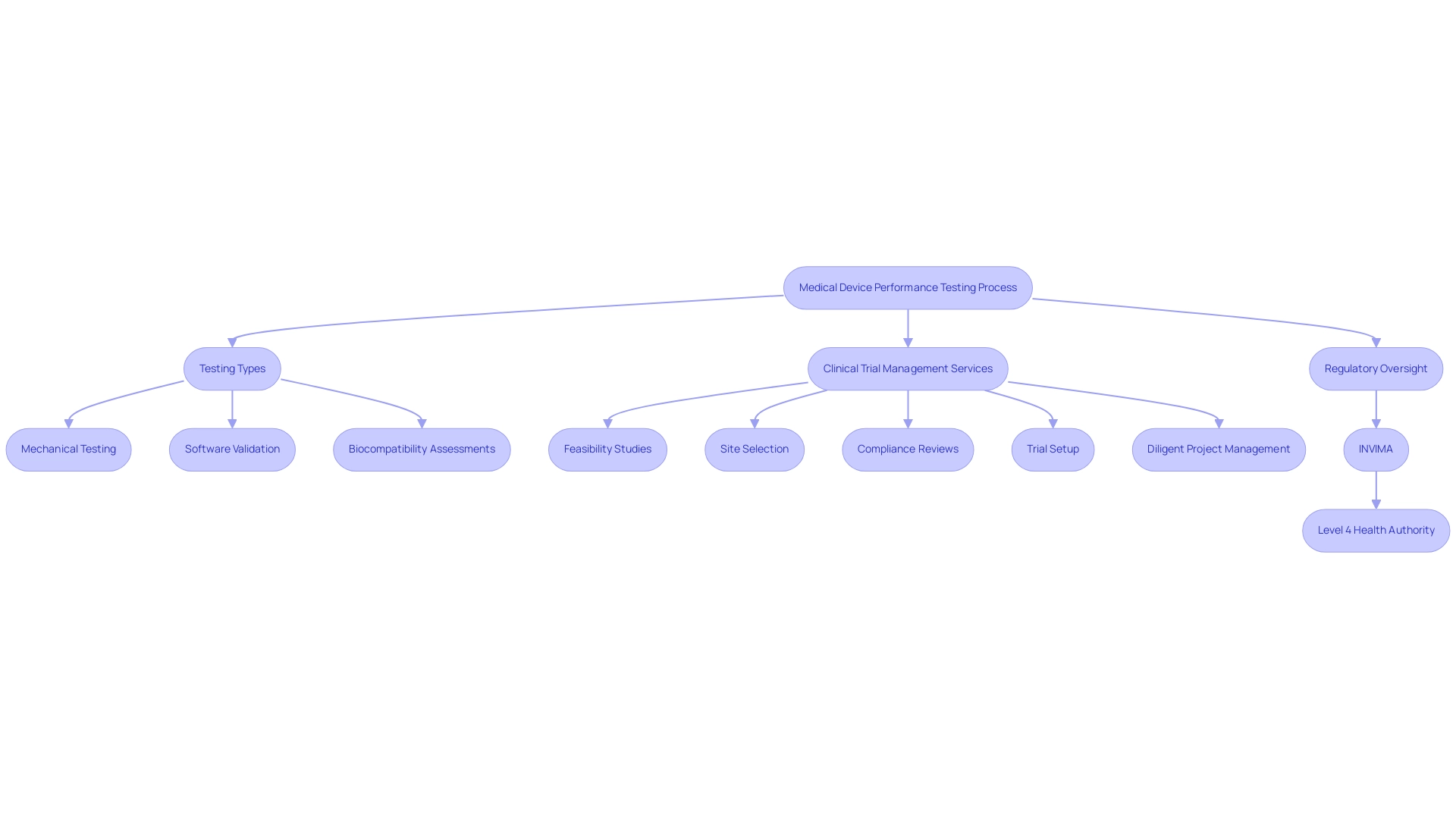
Medical device performance testing signifies a thorough and methodical method for assessing the functionality, reliability, and security of medical instruments. This process includes a variety of evaluations aimed at determining how effectively the equipment performs its intended functions under defined conditions. Such assessments are imperative in medical device performance testing to ensure compliance with established clinical standards, ultimately safeguarding patient health and facilitating successful treatment outcomes.
The process of medical device performance testing typically includes:
- Mechanical testing
- Software validation
- Biocompatibility assessments
All of which contribute significantly to establishing the efficacy and safety profile of each product prior to market entry. To support these efforts, comprehensive clinical trial management services, such as those offered by bioaccess®, ensure a streamlined process that includes:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Diligent project management and reporting
Specifically, bioaccess® specializes in various types of studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Post-Market Clinical Follow-Up Studies (PMCF)
In this context, the oversight provided by INVIMA, the Colombia National Food and Drug Surveillance Institute, plays a crucial role as a Level 4 health authority recognized by PAHO/WHO, ensuring that healthcare products meet stringent regulatory requirements. The healthcare equipment market is expected to expand at a consistent rate of 5.19% each year, with a market volume projected to attain US$233.50 billion by 2029, highlighting the increasing demand for comprehensive medical device performance testing and clinical research services. Significantly, the Asia Pacific area is anticipated to show the greatest growth rate in the healthcare equipment assessment market during this time, indicating its developing healthcare framework and regulatory environment.
Furthermore, leading companies in the sector, such as TÜV SÜD and Bureau Veritas, have recently expanded their operations and made significant acquisitions, as evidenced by recent developments in the industry. As Brian Moore, VP of NICCA USA, Inc., states, > The quality of research they have done for us has been excellent <. This highlights the crucial role of strong performance evaluation methods, alongside thorough clinical trial management services, in ensuring the reliability and efficacy of healthcare products, which are essential components of medical device performance testing in the changing healthcare environment.
The Importance of Performance Testing in Medical Device Safety
Medical device performance testing is essential to the security of healthcare instruments, serving as a proactive approach to detect potential hazards and malfunctions before clinical application. In Colombia, the INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) plays a crucial role in this context, overseeing the marketing and manufacturing of health products and ensuring compliance with rigorous safety standards. As a Level 4 health authority classified by the Pan American Health Organization/World Health Organization, INVIMA is recognized for its competence and efficiency in performing health regulation functions.
The Directorate for Medical Equipment and other Technologies within INVIMA oversees and regulates medical instruments, tracks pre- and post-market programs, and recommends technical standards for manufacturing, marketing, surveillance, and quality assurance. Through standardized assessment protocols, manufacturers can rigorously evaluate their products to ensure they meet the stringent criteria set forth by INVIMA and other regulatory authorities. This meticulous process not only safeguards patients from harm but also bolsters the reputation of manufacturers within the competitive healthcare landscape.
Significantly, data shows that equipment design problems were the primary reason for recalls, representing 56 incidents, or 29.0%, of total recalls, with 23 of these categorized as class 1 recalls—an indicator of serious risk to patient well-being. Moreover, devices that undergo medical device performance testing and comprehensive evaluations are significantly less likely to face recalls or be linked to adverse events, fostering essential trust between healthcare providers and patients. Findings from the case study named 'Medical Device Recalls by Specialty' indicate that cardiovascular procedures experienced the highest number of recalls, emphasizing the essential need for thorough evaluation and oversight in high-incidence areas to guarantee patient well-being.
This connection is additionally highlighted by Dr. Ross, a participant of the Medicare Evidence Development & Coverage Advisory Committee, who stresses that 'thorough examination and monitoring are essential in high-incidence regions to guarantee patient well-being.' Considering recent recall data, where 0.47% of recalls involved valve displacement, it becomes evident that medical device performance testing is crucial for efficient evaluations, as it directly influences patient protection and the overall dependability of healthcare instruments, while also aiding in economic development and job generation within the healthcare industry.
Exploring Different Types of Medical Device Performance Testing
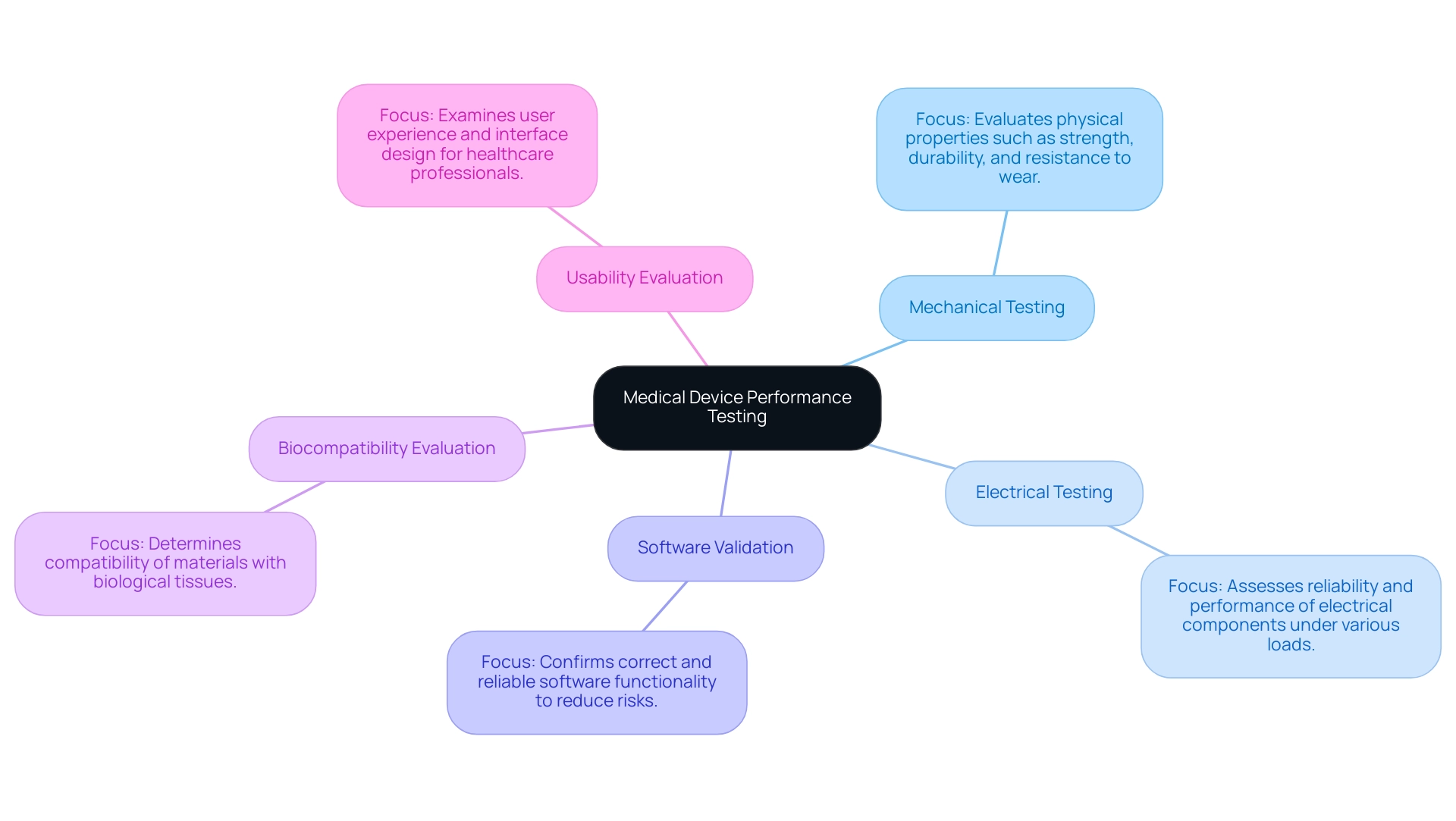
Medical device performance testing is a multifaceted process that encompasses various types of evaluations, each focusing on specific functionalities crucial for safety and efficacy. The primary categories include:
- Mechanical Testing: This type evaluates the physical properties of medical instruments, including their strength, durability, and resistance to wear and tear. It is essential in forecasting how equipment will operate under real-world conditions.
- Electrical Testing: Crucial for apparatus with electrical parts, this testing assesses their reliability and performance under different electrical loads. Ensuring electrical integrity is vital to prevent malfunctions that could jeopardize patient safety.
- Software Validation: Given the increasing integration of software in medical equipment, this process confirms that the software functions correctly and reliably. It reduces risks linked to software failures, which can have serious consequences for performance.
- Biocompatibility Evaluation: This assessment determines the compatibility of material substances with biological tissues, ensuring that no adverse reactions occur upon contact with the human body. This aspect is crucial for instruments intended for implantation or extended use.
- Usability Evaluation: Centered on the user experience, this assessment examines how effortlessly healthcare professionals can utilize the equipment. It involves evaluating the user interface design and overall interaction to ensure that the product is intuitive and user-friendly.
These varied assessment approaches in medical device performance testing guarantee a thorough evaluation of all performance aspects, thereby improving the safety and efficacy of healthcare instruments. As emphasized by Cigniti, an ISO 13485:2016 certified organization that offers software evaluation services for large healthcare equipment producers, "the integration of multidisciplinary research teams—engaging physician specialists and data scientists—has proven essential in enhancing outcome selection and data extraction methods." Such collaboration ultimately improves the quality and significance of research, which is increasingly acknowledged as essential for advancing equipment standards.
For example, a recent case analysis showed that involving multidisciplinary teams greatly enhanced the results of healthcare equipment evaluations, highlighting the significance of joint efforts in attaining high evaluation standards.
How Medical Device Performance Testing Works
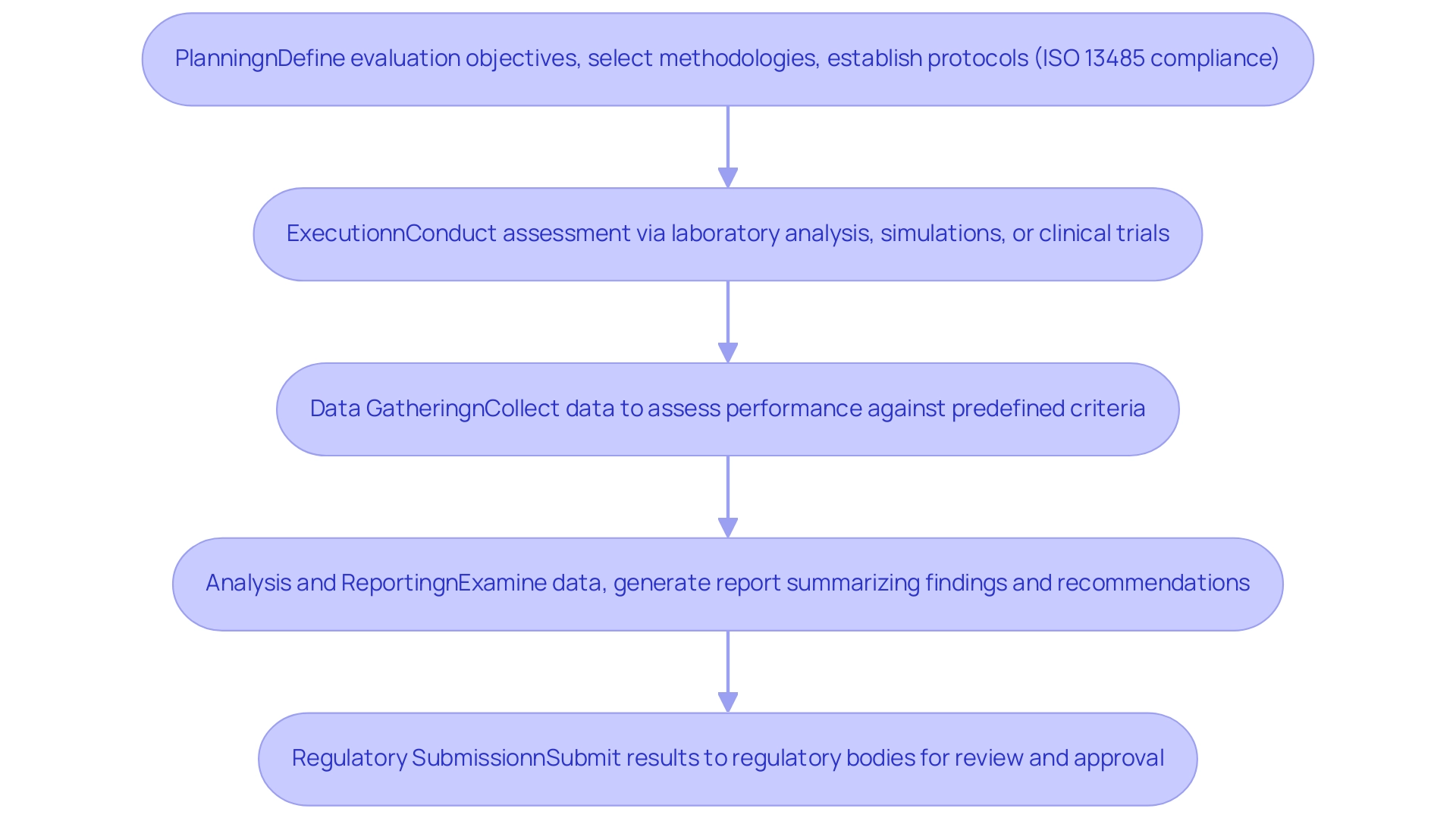
The medical device performance testing process is an intricate procedure that follows several key steps to ensure compliance with rigorous safety and effectiveness standards:
- Planning: This foundational phase involves defining clear evaluation objectives, selecting suitable methodologies, and establishing protocols that align with regulatory requirements, such as ISO 13485 compliance, which is crucial for quality management systems in healthcare products. According to Nicole Sheynin, a Content Marketing Specialist, "A well-organized planning phase is crucial for the successful implementation of medical device performance testing, as it establishes the foundation for all following activities."
- Execution: During this phase, the actual assessment takes place according to the established protocols. This may involve laboratory analysis, simulations, or clinical trials, depending on the kind of equipment and its intended application. Recent case studies highlight varied execution methods tailored to specific characteristics and regulatory expectations. For example, the case study named 'Design Elements in Biomaterial Development' examines how design factors affect the planning stage of medical instrument performance evaluation.
- Data Gathering: Throughout the evaluation process, meticulous data collection is vital. This data is collected for medical device performance testing to assess the performance of the apparatus against predefined criteria, forming the basis for subsequent analysis. Industry experts underscore the importance of this phase, noting that comprehensive data collection is essential for accurate medical device performance testing and regulatory compliance. Statistics show that almost 70% of medical equipment evaluation processes face delays due to inadequate data gathering practices, emphasizing the necessity for attentiveness in this field. bioaccess® addresses these challenges by providing thorough feasibility studies and site selection, ensuring that data collection processes are optimized from the outset.
- Analysis and Reporting: Upon completion of testing, the collected data is thoroughly examined to determine whether the apparatus meets established performance and quality standards. A detailed report is then generated, summarizing the findings, methodologies employed, and any recommendations for improvements to the equipment. This reporting phase is essential, as it aids in the ongoing monitoring and post-market clinical follow-up studies that bioaccess® oversees, ensuring continued adherence to regulatory standards.
- Regulatory Submission: The final step involves submitting the results to relevant regulatory bodies, such as INVIMA in Colombia, for review and approval. INVIMA plays a crucial role in oversight and classification of equipment as a Level 4 health authority by PAHO/WHO, ensuring that products comply with industry standards before they can be marketed. Notably, the current landscape has seen challenges such as project delays and modifications that do not align with legislative changes in the EU, making this submission process more critical than ever. bioaccess® provides support in trial setup and compliance reviews to navigate these regulatory complexities effectively.
These steps demonstrate a thorough method for medical device performance testing, ensuring that the instruments are rigorously assessed for reliability, effectiveness, and adherence to regulatory standards prior to entering the market. By utilizing knowledge in early feasibility, first-in-human, and pivotal studies, alongside customized project management and reporting services, organizations can navigate the complexities of clinical trials and improve their likelihood of successful market entry.
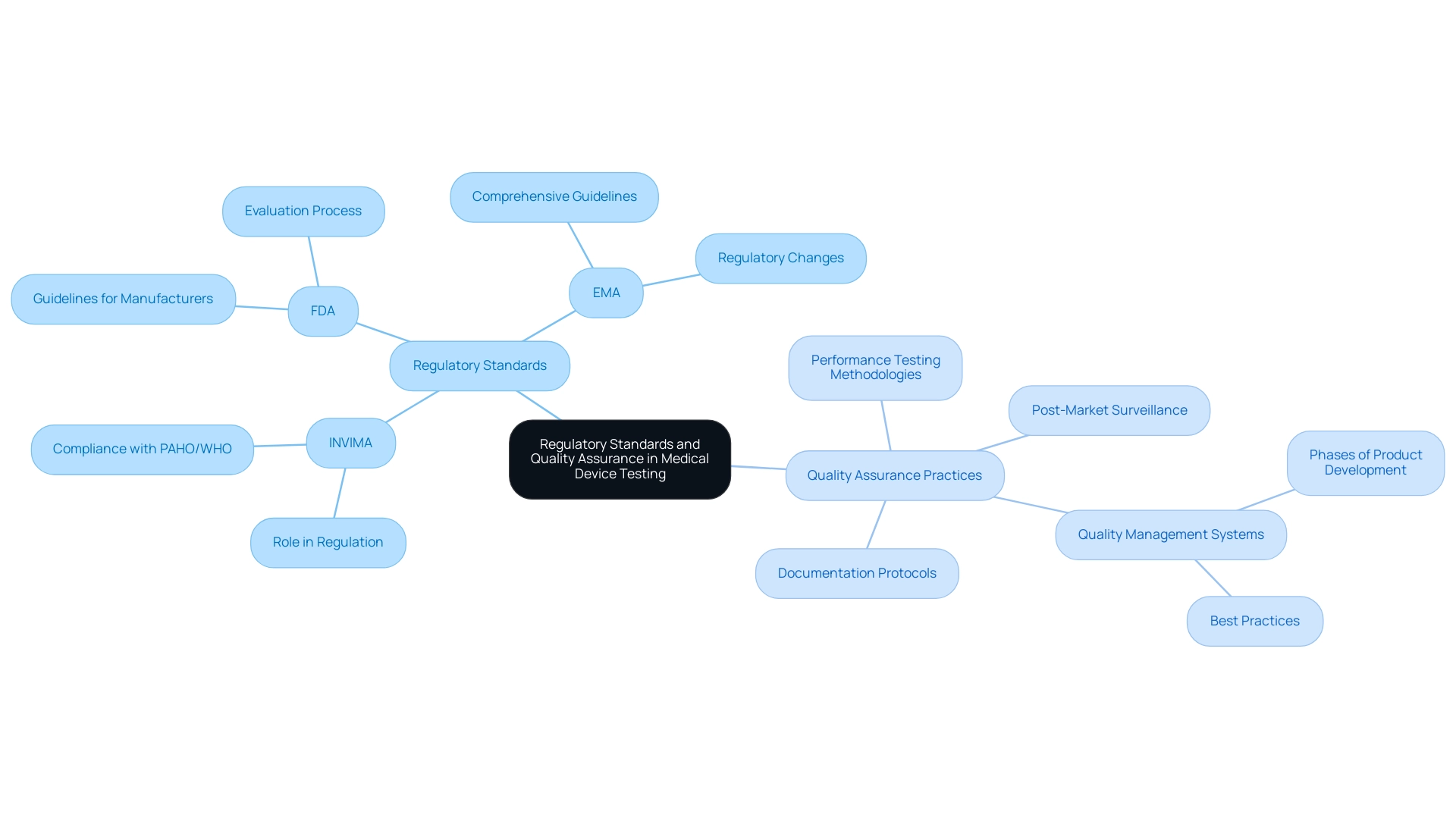
Regulatory Standards and Quality Assurance in Medical Device Testing
Regulatory standards are essential to guaranteeing the performance and safety of healthcare products, which necessitates medical device performance testing to ensure that all items meet strict criteria for safety and efficacy before market introduction. In Colombia, the National Food and Drug Surveillance Institute (INVIMA) plays a critical role as a Level 4 health authority, overseeing medical device regulation and ensuring compliance with international standards established by organizations such as the PAHO/WHO. Prominent regulatory bodies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), also create comprehensive guidelines that manufacturers must follow during the evaluation process.
These guidelines encompass various critical components, such as:
- Medical device performance testing methodologies
- Documentation protocols
- Post-market surveillance requirements
Furthermore, Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, emphasizes the importance of aligning with these regulations to ensure market readiness. In conjunction with regulatory frameworks, the implementation of effective quality assurance practices is vital for preserving the integrity of the assessment process.
Manufacturers are mandated to adopt robust quality management systems (QMS) that span all phases of product development, from initial design and testing through to production and distribution. This guarantees that all healthcare instruments not only adhere to regulatory standards but also conform to best practices in quality assurance. By following these strict regulations and applying comprehensive quality assurance measures, manufacturers can confidently assure that their healthcare products meet the standards of medical device performance testing to ensure they are both safe for patient use and effective in clinical applications.
Moreover, recent discussions highlight that the agreement between various assessment methodologies can vary significantly based on the demographics of the subjects involved, even when the performance of the tests remains consistent. This highlights the importance of continuous vigilance in adhering to FDA and EMA regulations, especially as they change to tackle new challenges in product evaluation. For instance, if the Reliability Quality Level (RQL) is set at 2, it is essential to note that the sample size (n) can be rounded up to 6 years, providing insight into the timelines involved in testing.
As emphasized by Dr. Wayne A. Taylor, 'This book is designed for engineers and scientists in the healthcare equipment field,' the significance of thorough standards and their careful implementation cannot be overstated. Furthermore, the case study titled 'Corrections in First Printing' illustrates how addressing errors in documentation can significantly enhance the accuracy and reliability of medical product information. Manufacturers must ensure they are not only compliant but also proactive in refining their quality assurance practices, especially through medical device performance testing, to enhance patient safety and product efficacy.
To effectively navigate these regulatory landscapes, our service capabilities include:
- Thorough feasibility studies and site selection to identify the most suitable research sites and principal investigators (PIs)
- Comprehensive reviews and feedback on study documents to ensure compliance with country-specific requirements
- Trial set-up, start-up, and obtaining necessary approvals from ethics committees and health ministries
- Assistance with import permits and the nationalization of investigational devices
- Efficient project management and monitoring activities
Our reporting services cover study status updates, inventory management, and documentation of both serious and non-serious adverse events, reinforcing our commitment to quality assurance and compliance throughout the clinical trial process.
Conclusion
The performance testing of medical devices is an indispensable component of healthcare, ensuring that devices are not only effective but also safe for patient use. Through a systematic approach that includes:
- Mechanical testing
- Electrical testing
- Software testing
- Biocompatibility testing
manufacturers can rigorously evaluate their products against established clinical standards. This meticulous process is further supported by regulatory bodies such as INVIMA in Colombia, which oversees compliance and safety standards in the medical device sector.
As the medical devices market expands, so too does the necessity for stringent performance evaluations. The increasing complexity of medical technology demands a robust framework for testing that encompasses both pre-market assessments and ongoing post-market surveillance. By adhering to established regulatory standards and implementing comprehensive quality assurance practices, manufacturers can foster trust among healthcare providers and patients, ultimately enhancing the reputation of their products in a competitive landscape.
In conclusion, the significance of thorough performance testing cannot be overstated. It directly impacts not only patient safety but also the overall reliability of medical devices, thereby contributing to the growth and sustainability of the healthcare sector. As the industry continues to evolve, the commitment to rigorous testing and adherence to regulatory frameworks will remain critical in ensuring that medical devices meet the highest standards of safety and efficacy, securing improved health outcomes for patients worldwide.
Frequently Asked Questions
What is medical device performance testing?
Medical device performance testing is a thorough and methodical process used to assess the functionality, reliability, and safety of medical instruments. It involves various evaluations to determine how effectively the equipment performs its intended functions under defined conditions.
Why is medical device performance testing important?
It is essential for ensuring compliance with established clinical standards, safeguarding patient health, and facilitating successful treatment outcomes. It also helps detect potential hazards and malfunctions before clinical application.
What types of evaluations are included in medical device performance testing?
The process typically includes mechanical testing, software validation, and biocompatibility assessments, all of which contribute to establishing the efficacy and safety profile of each product prior to market entry.
What services does bioaccess® provide to support medical device performance testing?
Bioaccess® offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, and diligent project management and reporting.
What types of studies does bioaccess® specialize in?
Bioaccess® specializes in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Post-Market Clinical Follow-Up Studies (PMCF).
What role does INVIMA play in medical device performance testing in Colombia?
INVIMA, the Colombian National Food and Drug Surveillance Institute, oversees the marketing and manufacturing of health products, ensuring compliance with rigorous safety standards as a Level 4 health authority recognized by PAHO/WHO.
What are the consequences of equipment design problems in medical devices?
Equipment design problems are a primary reason for recalls, with data showing they account for 29.0% of total recalls. Devices that undergo rigorous performance testing are significantly less likely to face recalls or be linked to adverse events.
How does medical device performance testing influence patient safety?
Thorough evaluations and oversight help ensure that medical devices are safe and reliable, which fosters trust between healthcare providers and patients, ultimately protecting patient well-being.
What is the projected growth of the healthcare equipment market?
The healthcare equipment market is expected to expand at a rate of 5.19% per year, with a projected market volume of US$233.50 billion by 2029.
Which region is anticipated to show the greatest growth rate in the healthcare equipment assessment market?
The Asia Pacific area is expected to exhibit the highest growth rate due to its developing healthcare framework and regulatory environment.

