Introduction
In the realm of clinical research, pivotal studies serve as a fundamental element in establishing the efficacy and safety of medical products, shaping the future of healthcare innovation. These meticulously designed trials are not only essential for regulatory approval but also reflect the complexities faced by Medtech companies, particularly in regions like Latin America.
As these organizations navigate regulatory hurdles, language barriers, and resource fragmentation, the significance of well-executed pivotal studies becomes increasingly apparent. They not only influence drug approvals and regulatory decisions but also underscore the importance of collaboration and strategic planning in overcoming the multifaceted challenges of clinical trials.
This article delves into the critical role of pivotal studies, exploring their characteristics, impact on drug approval processes, and the unique challenges encountered in their execution, ultimately highlighting the ongoing commitment to advancing medical interventions in a rapidly evolving landscape.
Defining Pivotal Studies: An Overview
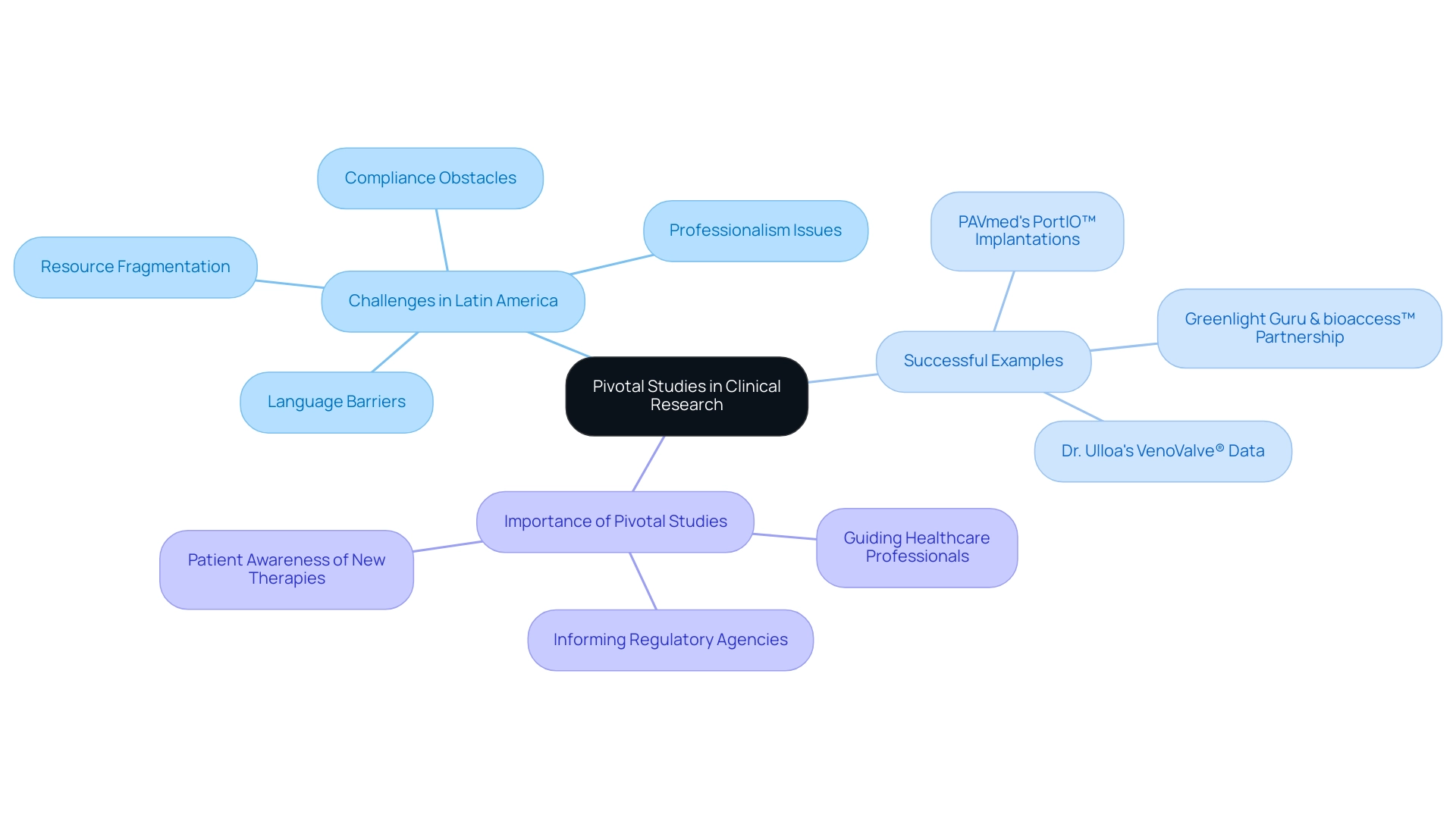
Crucial investigations embody the pivotal study meaning and serve as a foundation of clinical research, carefully crafted to produce the essential evidence backing the effectiveness and safety of medical products, such as medications and medical devices, for approval by authorities. These studies are generally large-scale and rigorously controlled, aiming to demonstrate a statistically significant difference between treatment and control groups, which helps clarify the pivotal study meaning. In the context of Latin America, Medtech companies encounter distinct challenges, including:
- Compliance obstacles
- Language barriers
- Professionalism issues
- Resource fragmentation
These challenges can hinder the smooth execution of these investigations.
Significantly, crucial experiments in cancer may have enrollment phases lasting from two to three years, emphasizing the considerable dedication and resources required in such research. The results of crucial research, which embody the pivotal study meaning, are essential as they inform regulatory agencies, healthcare professionals, and patients about the advantages and dangers linked to new therapies. For instance, advancements such as PAVmed's first-in-human implantations of the PortIO™ Intraosseous Infusion System in Colombia and Dr. Jorge Hernando Ulloa's presentation of one-year VenoValve® data at the Charing Cross International Symposium exemplify successful outcomes in this landscape.
Furthermore, partnerships, like the one between Greenlight Guru and bioaccess™, demonstrate creative methods to expedite Medtech innovations and clinical studies in the region, achieving over 50% reduction in recruitment time and 95% retention rates. This highlights the pivotal study meaning of key research in influencing the future of medical interventions while demonstrating a growing commitment to environmental, social, and governance principles in their implementation. It is essential to adopt a solution-driven approach to bridge the gap between innovation and execution, addressing the challenges that hinder progress in clinical research in Latin America.
The Role of Pivotal Studies in Clinical Research and Trial Design
Crucial investigations are essential to the structure of clinical research and experimental design, supplying the strong evidence required for compliance submissions. These investigations play a crucial role in determining trial design parameters such as sample size, endpoints, and statistical analysis methods. Each crucial research is meticulously crafted to align with regulatory standards while addressing specific queries posed by health authorities.
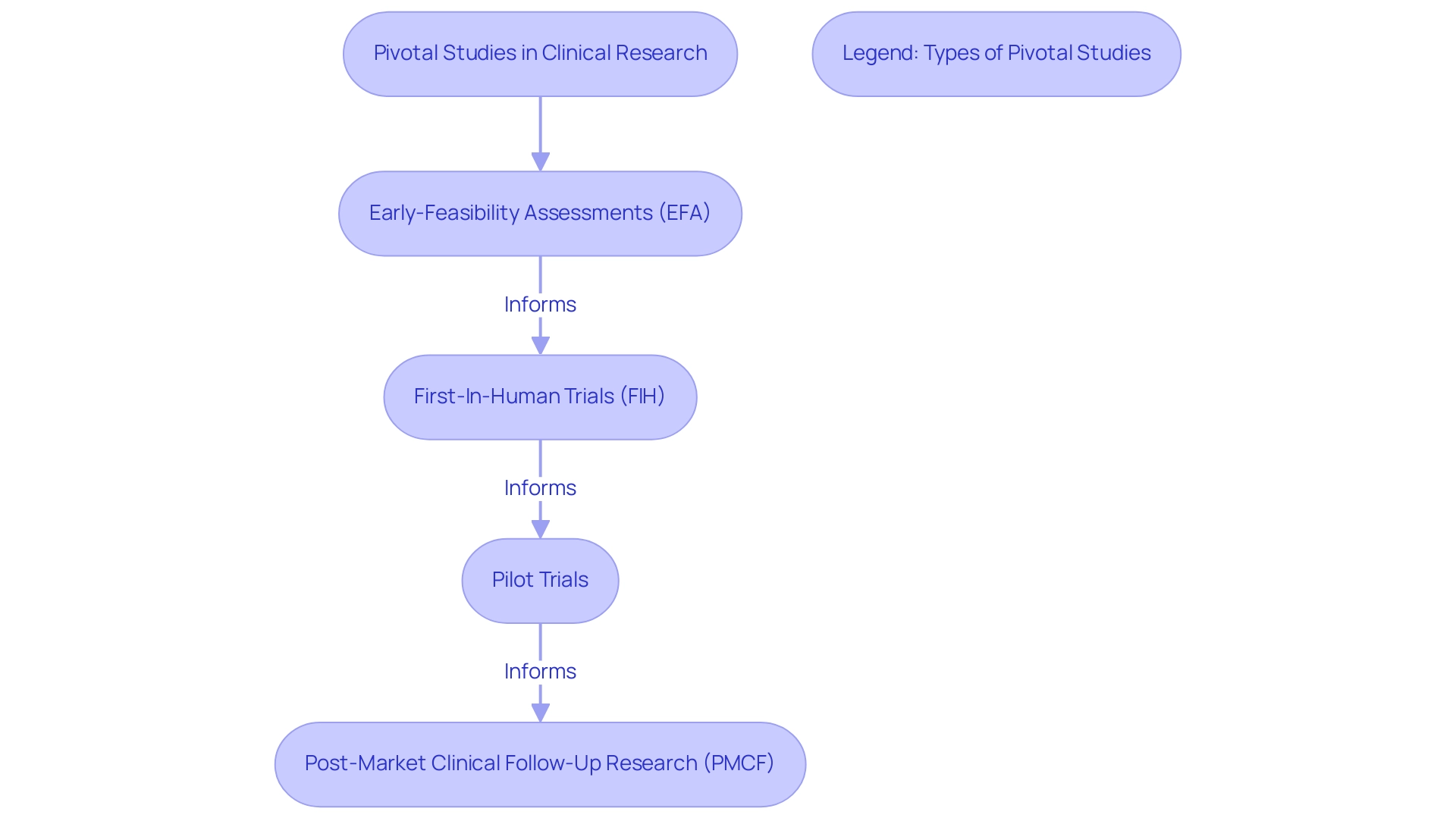
Utilizing over 20 years of expertise, bioaccess® specializes in managing crucial research, including:
- Early-Feasibility Assessments (EFA)
- First-In-Human Trials (FIH)
- Pilot Trials
- Post-Market Clinical Follow-Up Research (PMCF)
Our team's extensive experience and flexibility ensure that each assessment meets the highest compliance standards and is tailored to the specific needs of our clients. The results of these studies not only directly affect the design of subsequent experiments but also shape post-marketing surveillance initiatives and phase IV studies, creating a seamless continuum of research that ensures the ongoing assessment of a product’s safety and efficacy.
As Trudo Lemmens, a recognized authority in health law and policy, points out, the convergence of law and ethical principles in health product development is essential, stressing the necessity for thorough study designs that satisfy both oversight requirements and ethical benchmarks. Recent analysis of 354 medical dossiers connected to 117 devices illustrates the significant characteristics of crucial clinical trials, showcasing their essential role in the regulatory landscape. This analysis uncovers that crucial research often illustrates the complexities and challenges sponsors encounter in justifying their product's success, particularly in a competitive environment where comparable products may have failed.
Notably, the FDA has approved drugs based solely on the results of a single pivotal study meaning, particularly in the realm of cancer therapies, which highlights the immense responsibility placed on sponsors to justify the efficacy of their products amidst a competitive landscape where others may have faltered.
Key Characteristics of Pivotal Studies
The concept of pivotal study meaning is characterized by its rigorous methodological framework, which includes large sample sizes and the implementation of control groups to ensure robust results. A fundamental aspect of this research is its randomized and double-blinded design, which serves to minimize bias and bolster the credibility of the findings. The significance of randomization cannot be overstated; it is crucial for ensuring that the results are generalizable and that any observed effects can be attributed to the treatment itself.
Each crucial research is built around distinctly outlined primary and secondary endpoints, vital for precisely assessing the efficacy and safety of the intervention. This structured approach facilitates comprehensive data collection, integral for regulatory review processes, such as those conducted by the FDA. Significantly, the success of crucial research has been reflected in their impact on drug approvals, with 13 medications gaining FDA approval based on the achievement of primary endpoints from these investigations.
As highlighted in the literature, individual critical experiments with limited supporting features have been utilized for FDA approval of cancer therapies, emphasizing the significance of these investigations in therapeutic development. Moreover, bioaccess® focuses on overseeing crucial research and other clinical evaluation types, including:
- Early-Feasibility Assessments (EFA)
- First-In-Human Evaluations (FIH)
- Pilot Evaluations
- Post-Market Clinical Follow-Up Assessments (PMCF)
With more than 20 years of experience in Medtech, bioaccess® guarantees compliance, efficient site selection, and thorough project management throughout the testing process.
Furthermore, the case analysis on the combination of P-values from independent experiments demonstrates a flexible approach for researchers to maintain control over the overall Type-I error rate while achieving specific limits on the partial Type-I error rate. As the landscape of clinical research evolves, grasping the pivotal study meaning and key characteristics of pivotal trials remains vital for advancing therapeutic development.
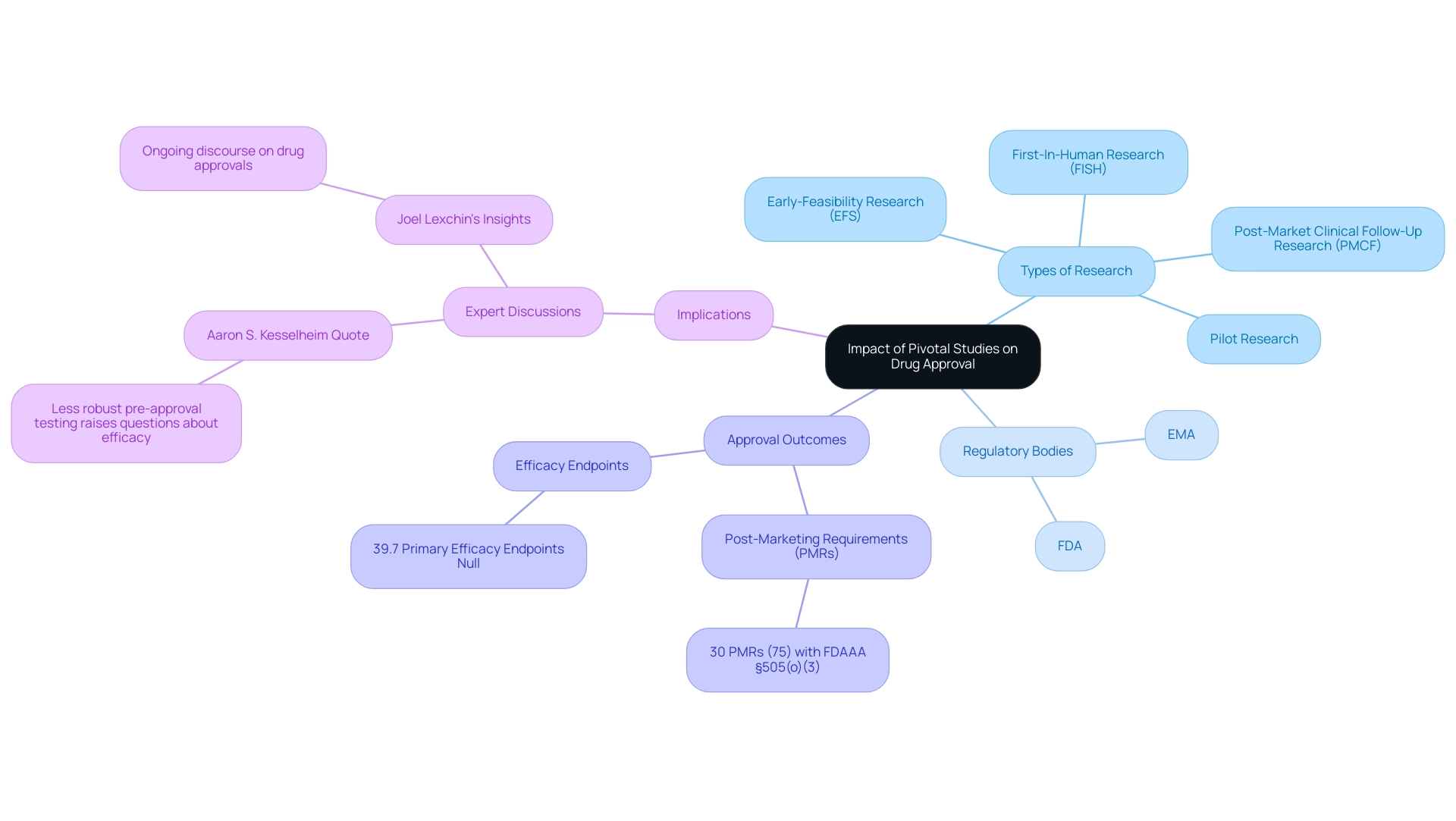
Impact of Pivotal Studies on Drug Approval and Regulatory Decisions
Crucial research is essential in the drug approval process, as the data generated from these trials serve as the foundation for submissions to regulatory bodies like the FDA and EMA. These analyses are meticulously evaluated to determine if the advantages of a new treatment outweigh the associated risks, highlighting the need for a balance between expedited drug approval and sufficient evidence of safety and efficacy. In fact, data reveal that 30 out of 40 Post-Marketing Requirements (PMRs)—a notable 75%—had a FDAAA §505(o)(3) PMR, indicating the intense scrutiny these evaluations undergo.
Successful critical investigations can expedite the approval process, while unsuccessful ones frequently result in delays or outright rejections. As highlighted by Aaron S. Kesselheim, the author and regulatory expert, 'Less robust pre approval testing raises questions about the efficacy and clinical value of these drugs.' This concern highlights the necessity for a pivotal study meaning high-quality essential research, which is crucial in protecting public health by determining which therapies are ultimately provided to patients.
At bioaccess®, we utilize our 20+ years of expertise in managing essential research, including:
- Early-Feasibility Research (EFS)
- First-In-Human Research (FISH)
- Pilot Research
- Post-Market Clinical Follow-Up Research (PMCF)
ensuring compliance and meticulous project management throughout the process. The case examination titled 'Characteristics of Drugs Approved with Null Findings' further illustrates this dynamic, revealing that 55 crucial trials supported approvals, despite 39.7% of primary efficacy endpoints being null. The research identified characteristics such as the types of drugs involved, the expedited pathways utilized, and the therapeutic areas targeted.
Typical reasons for these approvals encompassed success in other crucial research and positive post hoc evaluations, thus illustrating the subtle influence of pivotal study meaning on decision-making by authorities. Additionally, as noted by Joel Lexchin, the ongoing discourse around the implications of these findings remains critical in understanding the evolving landscape of drug approvals. Our emphasis on delivering expedited medical device clinical research services in Latin America enables us to maneuver through the distinct compliance landscape of the area, leveraging our specialized expertise and adaptability to satisfy the needs of our clients.
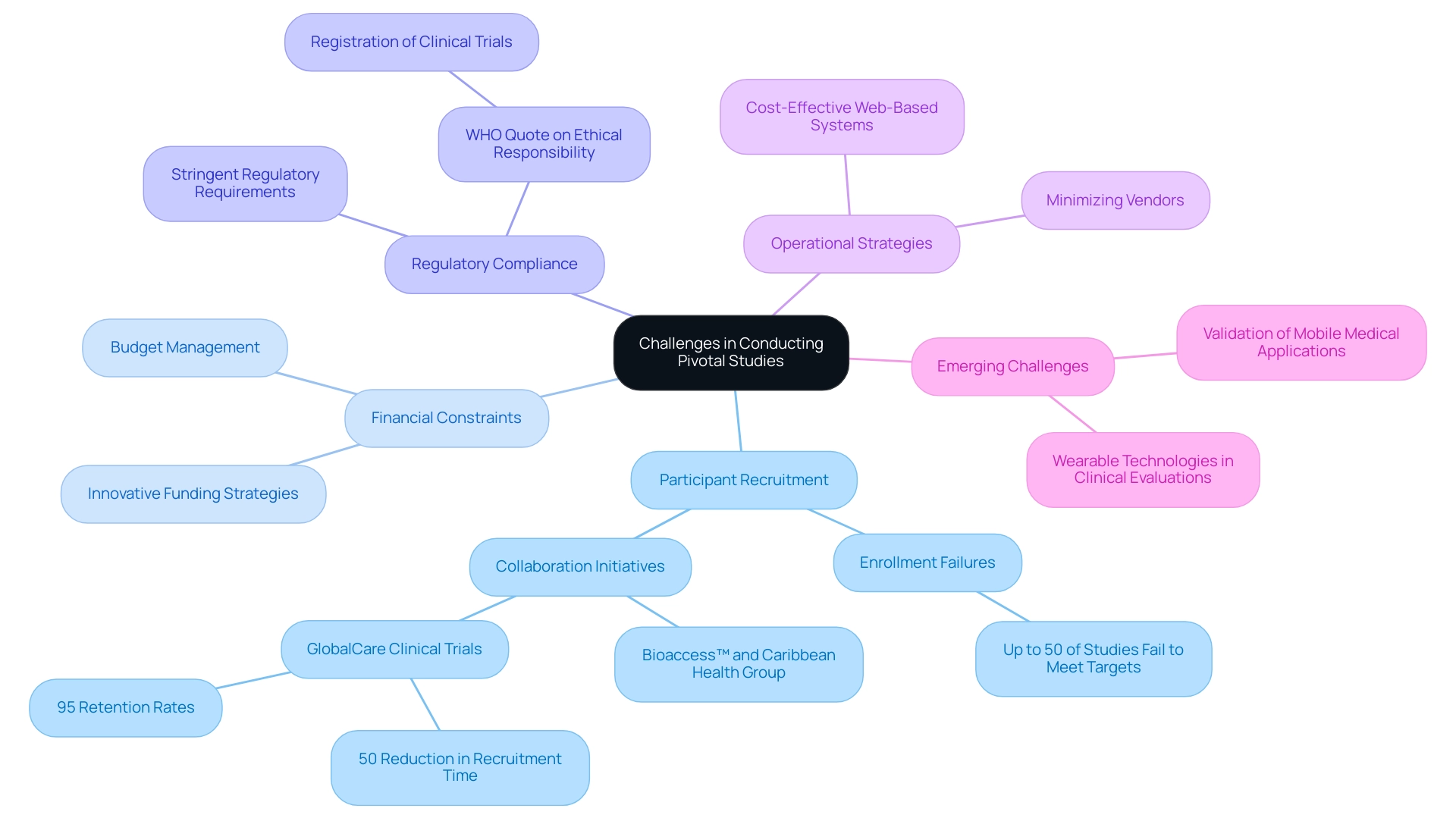
Challenges in Conducting Pivotal Studies
Conducting crucial research is fraught with challenges, particularly in the areas of participant recruitment, financial constraints, and regulatory compliance. Recruitment difficulties often arise when targeting a sufficient number of eligible participants, especially in studies focused on rare diseases or conditions. Recent statistics indicate that recruitment challenges significantly hinder progress, with up to 50% of studies failing to meet their target enrollment numbers.
This issue is compounded by initiatives such as the collaboration between bioaccess™ and the Caribbean Health Group, supported by Colombia's Minister of Health, aimed at positioning Barranquilla as a leading destination for clinical studies in Latin America. This strategic collaboration seeks to streamline the feasibility and selection of research sites and principal investigators (PIs), enhance trial set-up processes, and improve project management capabilities, ultimately bolstering recruitment efforts. Financially, the burden of executing large-scale studies can strain resources, prompting the need for meticulous budget management and innovative funding strategies.
Furthermore, adherence to stringent regulatory requirements adds complexity; researchers must navigate a labyrinth of diverse guidelines to ensure ethical compliance. As the World Health Organization emphasizes, 'the registration of a clinical study is a scientific, ethical, and moral responsibility.' Effectively addressing these challenges requires strategic planning, collaboration with stakeholders, and a steadfast commitment to upholding the highest standards of research integrity.
In this context, notable collaborations such as GlobalCare Clinical Trials partnering with bioaccess™ have achieved over a 50% reduction in recruitment time and maintained 95% retention rates, showcasing the potential benefits of such alliances. To optimize operational efficiency, minimizing the number of vendors and utilizing cost-effective web-based systems can be beneficial. Moreover, recent presentations, like 'Challenges in Validating Mobile Medical Applications,' highlight the changing environment of medical testing and the distinct obstacles faced in validating mobile medical devices and wearable technologies, which are becoming increasingly significant in clinical evaluations, thus illustrating the wider challenges experienced in critical examinations.
Reporting on study status and adverse events is also critical to ensure transparency and adherence to regulatory standards, further enhancing the credibility of the trial outcomes.
Conclusion
The examination of pivotal studies reveals their indispensable role in clinical research, particularly in establishing the safety and efficacy of medical products. These meticulously designed trials not only provide the critical evidence needed for regulatory approvals but also navigate the unique challenges inherent to regions like Latin America. From regulatory hurdles to language barriers, the complexities faced by Medtech companies emphasize the need for strategic planning and collaboration to ensure successful outcomes.
Key characteristics of pivotal studies, including their rigorous methodologies and adherence to ethical standards, underscore their significance in the drug approval process. The ability of these studies to influence regulatory decisions highlights their essential function in safeguarding public health. Furthermore, the challenges encountered in conducting these trials, from recruitment difficulties to financial constraints, necessitate innovative solutions and partnerships to streamline operations and enhance efficiency.
Ultimately, the commitment to advancing medical interventions through well-executed pivotal studies is vital for the future of healthcare innovation. As the landscape of clinical research continues to evolve, the ongoing dedication to overcoming challenges and fostering collaboration will pave the way for meaningful advancements in medical therapies, ultimately benefiting patients and healthcare providers alike. The path forward hinges on a collective effort to uphold the highest standards of research integrity while embracing the opportunities that lie ahead in the realm of clinical trials.
Frequently Asked Questions
What are crucial investigations in clinical research?
Crucial investigations are pivotal studies that provide essential evidence for the effectiveness and safety of medical products, such as medications and medical devices, needed for approval by regulatory authorities.
What is the purpose of pivotal studies?
Pivotal studies aim to demonstrate a statistically significant difference between treatment and control groups, thereby clarifying the effectiveness and safety of medical interventions.
What challenges do Medtech companies face in Latin America?
Medtech companies in Latin America encounter challenges such as compliance obstacles, language barriers, professionalism issues, and resource fragmentation, which can hinder the execution of clinical investigations.
How long can enrollment phases last for crucial experiments in cancer research?
Enrollment phases for crucial experiments in cancer research can last from two to three years, indicating the significant commitment and resources required for such studies.
Why are the results of crucial research important?
The results of crucial research inform regulatory agencies, healthcare professionals, and patients about the benefits and risks associated with new therapies, influencing medical decisions and product approvals.
Can you provide examples of successful outcomes in Medtech research?
Examples include PAVmed's first-in-human implantations of the PortIO™ Intraosseous Infusion System in Colombia and Dr. Jorge Hernando Ulloa's presentation of one-year VenoValve® data at the Charing Cross International Symposium.
How can partnerships improve Medtech innovations and clinical studies?
Partnerships, such as between Greenlight Guru and bioaccess™, have demonstrated innovative approaches that can reduce recruitment time by over 50% and achieve 95% retention rates in clinical studies.
What role does bioaccess® play in managing crucial research?
Bioaccess® specializes in managing crucial research, including Early-Feasibility Assessments, First-In-Human Trials, Pilot Trials, and Post-Market Clinical Follow-Up Research, ensuring compliance with regulatory standards.
How do crucial investigations impact post-marketing surveillance?
The results of crucial investigations shape post-marketing surveillance initiatives and phase IV studies, creating a continuum of research that ensures ongoing assessment of a product’s safety and efficacy.
What is the significance of thorough study designs in health product development?
Thorough study designs are essential to meet both regulatory oversight requirements and ethical benchmarks, as emphasized by health law experts like Trudo Lemmens.

