Introduction
The Premarket Approval (PMA) process stands as a cornerstone in the regulation of medical devices, particularly for those deemed high-risk due to their potential impact on patient safety. As the healthcare landscape continues to evolve, understanding the intricacies of PMA is crucial for manufacturers aiming to navigate the complexities of regulatory compliance.
This article delves into the significance of the PMA process, outlining its rigorous steps, key differences compared to the 510(k) pathway, and best practices for successful submissions. By examining the challenges and opportunities presented by the PMA framework, stakeholders can better prepare for the demands of ensuring device safety and efficacy, ultimately contributing to improved public health outcomes.
Understanding Premarket Approval (PMA): Definition and Importance
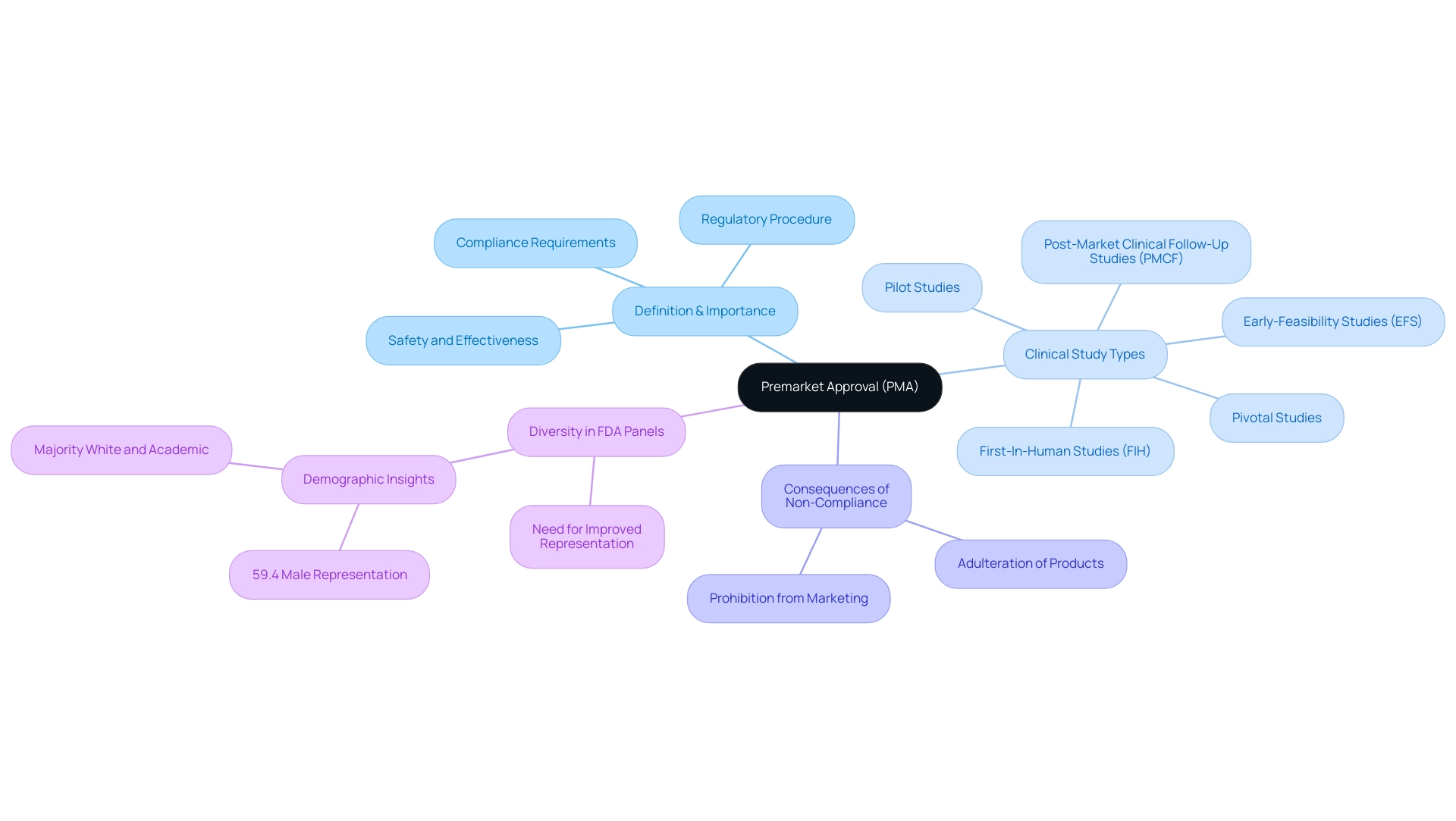
What is premarket approval (PMA)? It is a crucial regulatory procedure established by the FDA for the assessment of specific medical instruments, particularly those categorized as Class III, which are regarded as higher risk due to their potential effect on patient safety. This thorough procedure not only evaluates the safety and effectiveness of these products but also guarantees that they comply with the essential regulatory criteria before entering the market in the United States. Manufacturers must understand what is premarket approval, as it signifies adherence to regulatory requirements, which is essential for successful commercialization.
The PMA involves extensive scientific review, underscoring its importance in maintaining industry standards and safeguarding public health. In the context of accelerated medical equipment clinical study services, companies like bioaccess® provide comprehensive clinical trial management, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
With over 20 years of expertise in the Medtech field, bioaccess® ensures that these studies are designed to meet evolving regulatory expectations, emphasizing the flexibility and specialized knowledge required for successful clinical trials.
This customized approach is vital for navigating the complexities of what is premarket approval, as highlighted by Dr. Murad Alam, who noted a need for improved study designs and more relevant clinical data. Importantly, Class III products that do not meet what is premarket approval requirements are considered adulterated and prohibited from being marketed, further illustrating the critical nature of this approval pathway. Additionally, the demographic analysis of FDA panel members reveals a lack of diversity, with 59.4% of respondents being male and a majority being White and in academic practice, highlighting the need for a more representative decision-making body.
The changing environment of medical device regulation is further demonstrated by the number of COVID-19 treatment vaccine trials, which highlights the significance of prompt PMA procedures in meeting public health needs. Moreover, experts such as Juan Cuya, MD, specialized in Regulatory Affairs and health technology, enhance the overall success of these clinical studies, making the PMA approach more effective and aligned with current health challenges.
Navigating the PMA Process: Steps and Requirements
To understand what is premarket approval, it's essential to recognize that the PMA procedure encompasses several critical steps that require careful execution to meet FDA regulatory standards, and our comprehensive clinical trial management services can greatly support this endeavor. First, the Pre-Submission phase is vital; engaging early with the FDA can significantly aid in clarifying expectations and requirements for your product. Our group, directed by Katherine Ruiz, a specialist in regulatory matters for medical products and in vitro diagnostics in Colombia, is prepared to offer valuable advice during this proactive communication, which is promoted by the FDA to reduce delays during the application.
The FDA will publish a Federal Register notice of an order denying approval of the PMA after each quarter, underscoring the importance of thorough preparation. Next, in the Preparing the PMA Application phase, manufacturers must compile exhaustive data demonstrating what is premarket approval, including the product's safety and effectiveness, alongside detailed manufacturing methods and proposed labeling. Our services encompass reviewing and offering feedback on study documents to ensure compliance with country requirements, as well as facilitating the import permit procedures and nationalization of investigational devices, contributing to a robust submission.
Following preparation, the step of submitting the PMA addresses what is premarket approval, involving the formal presentation of the application to the FDA while ensuring that all required documentation is included. Once submitted, the application enters the FDA Review phase, where the agency meticulously evaluates the submission. As part of this procedure, the FDA intends to review the Post-Approval Study (PAS) protocol interactively with the sponsor during the evaluation of the PMA and concurrently with the assessment of the premarket data, providing a collaborative approach to ensure compliance.
Our study project management and monitoring services facilitate this interaction, ensuring all aspects are well-managed. This review may also involve convening an advisory committee to provide external expertise, further enhancing the review. Finally, the Decision step concludes the procedure; after thorough evaluation, the FDA will either approve or deny the PMA application, highlighting what is premarket approval.
It is imperative to note that if a PAS protocol is not developed by the time of PMA approval, an outline must be submitted within 30 days for FDA review. Furthermore, approval of a PMA application does not conclude FDA requirements; manufacturers must conduct post-approval studies to ensure ongoing safety and effectiveness of high-risk products. Each of these steps requires meticulous attention to detail, and our service capabilities are designed to ensure compliance and facilitate a smooth approval experience, including trial setup, project management, and comprehensive reporting on study status, inventory, and adverse events.
PMA vs. 510(k): Key Differences and Considerations
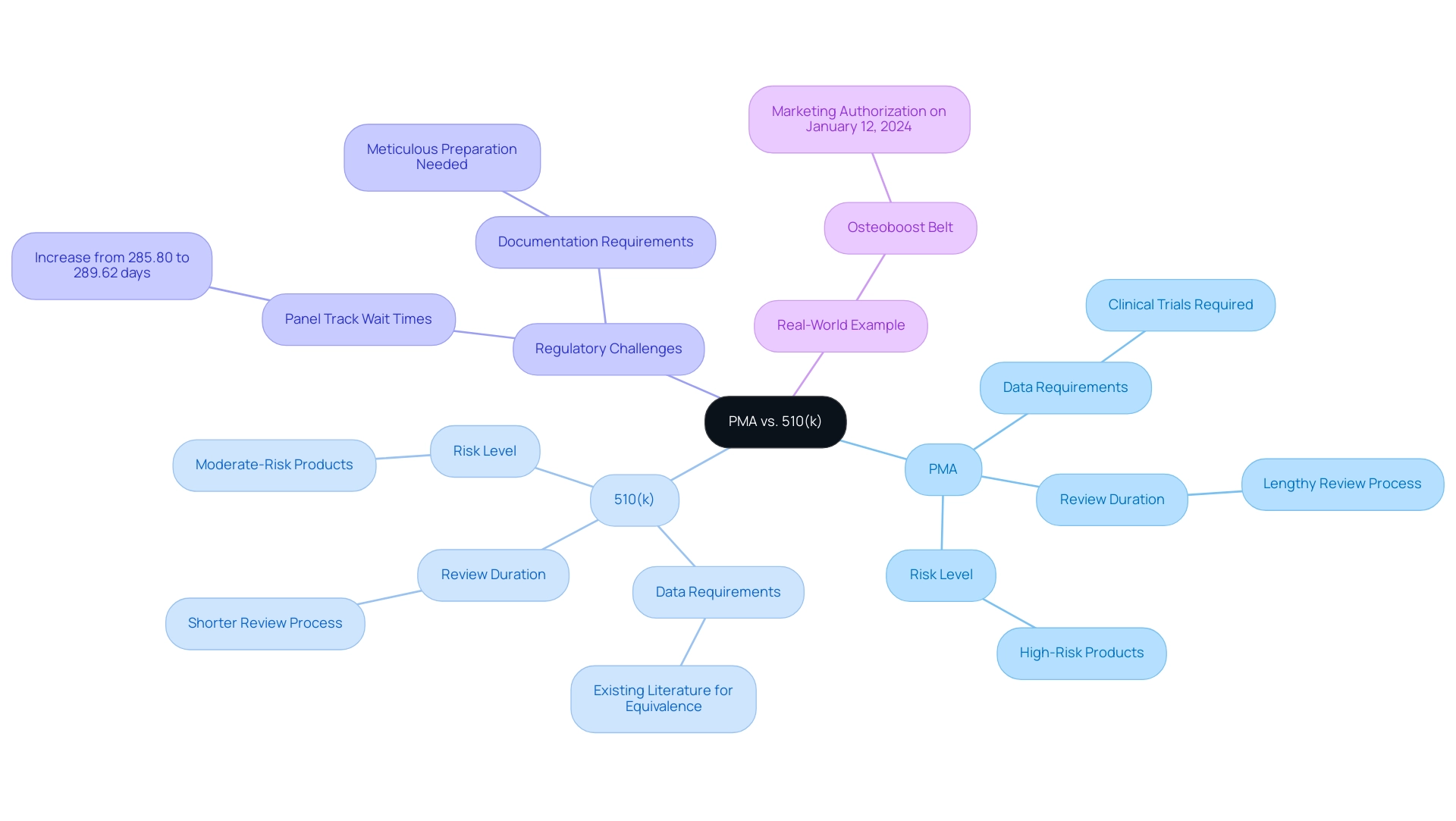
Understanding what is premarket approval is essential, as both the Premarket Approval (PMA) and the 510(k) procedures are crucial yet separate routes in the regulation of medical equipment, each addressing different risk levels linked to the products. Understanding what is premarket approval is essential for the PMA procedure for high-risk items, which necessitates extensive clinical data to support safety and efficacy assertions. In contrast, the 510(k) pathway is typically utilized for moderate-risk devices, permitting manufacturers to demonstrate that their device is substantially equivalent to an already marketed device.
Key distinctions between these methods are as follows:
- Data Requirements: PMA submissions necessitate the execution of clinical trials, while 510(k) applications may leverage existing scientific literature to establish equivalence.
- Review Duration: The PMA procedure generally involves a lengthier review period due to its rigorous evaluation standards.
- Risk Level: PMA is specifically designed for high-risk products, whereas the 510(k) route is reserved for those classified as moderate-risk.
Understanding these differences is critical for manufacturers, particularly in the context of what is premarket approval, as selecting the appropriate submission pathway can significantly impact the regulatory timeline and market entry strategy. Furthermore, careful preparation is necessary for the 510(k) submission, including thorough documentation of the product. Recent data indicates a slight increase in Panel Track wait times, which rose from an average of 285.80 days in 2023 to 289.62 days in 2024, reflecting ongoing challenges in the review process.
This insight emphasizes the importance of strategic planning in navigating the complexities of medical equipment regulation, particularly when utilizing the comprehensive clinical trial management services provided by experts like Katherine Ruiz. Her contributions involve managing feasibility studies, site selection, and trial setup, ensuring adherence to regulatory requirements for medical instruments and in vitro diagnostics in Colombia. Additionally, as highlighted by Nick Paul Taylor, 'BD receives FDA warning letter over quality system violations,' this serves as a reminder of the regulatory challenges manufacturers may face.
A real-world example is demonstrated with the Osteoboost Belt, for which BONE HEALTH TECHNOLOGIES, INC. received marketing authorization on January 12, 2024, providing insight into what is premarket approval and its implications in a regulated environment.
Timeline for PMA Approval: What to Expect
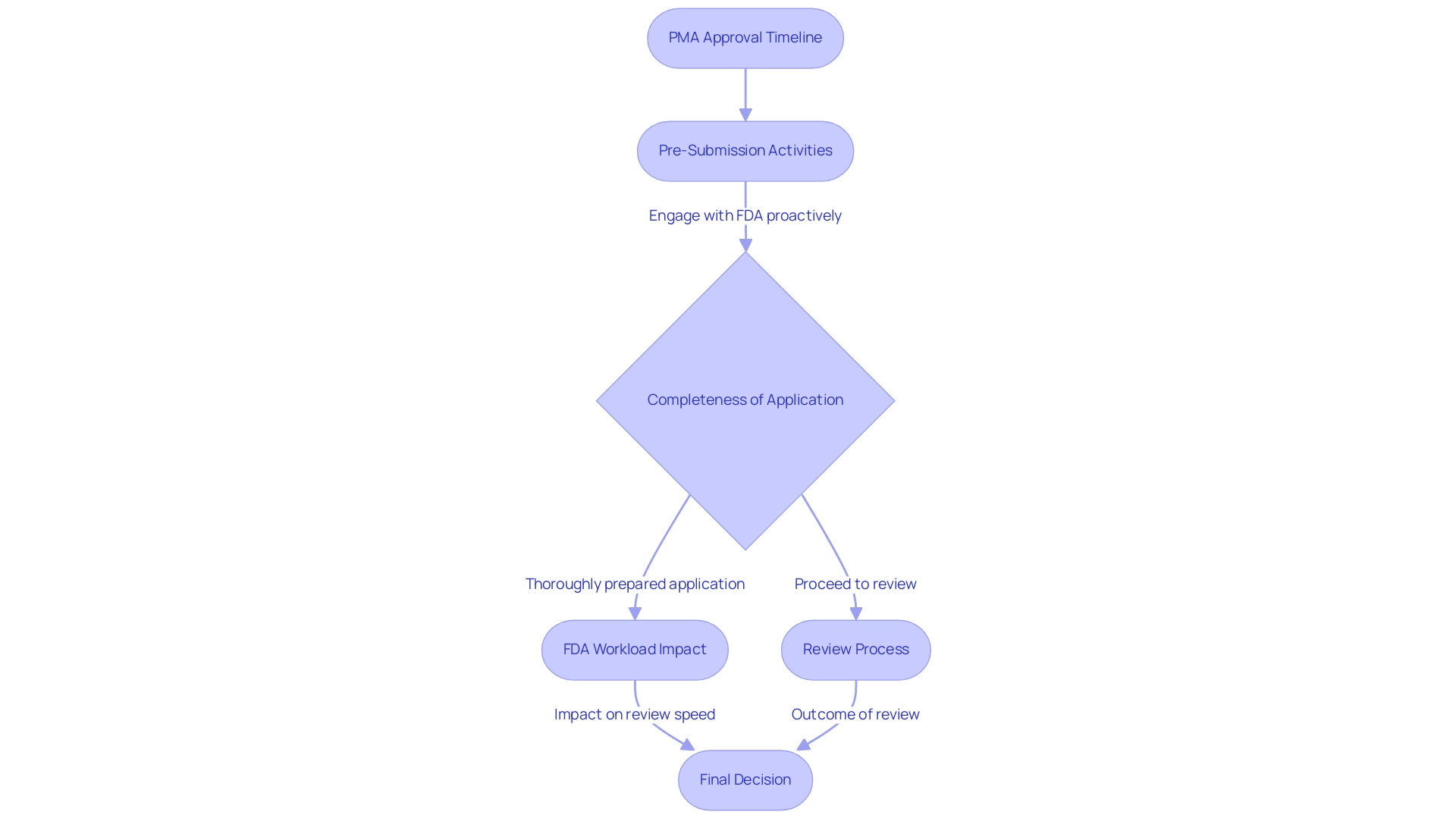
The timeline for medical devices, in the context of what is premarket approval (PMA), can differ widely, influenced by the complexity of the device and the quality of the submitted data. On average, the PMA procedure spans from 6 months to several years, reflecting significant variability. In 2024, for instance, the average wait times for Panel Track approvals increased slightly, rising from 285.80 days to 289.62 days, while De Novo wait times also saw a rise from 415 days to 420 days, highlighting the challenges faced in the regulatory landscape.
Comprehensive clinical trial management services, like those offered by bioaccess®, play a critical role in facilitating a smoother process of what is premarket approval. These services include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and detailed reporting. Several factors critically impact the timeline of what is premarket approval, including:
- Pre-Submission Activities: Engaging proactively with the FDA prior to submission can significantly streamline the review.
- Completeness of the Application: Submitting a thoroughly prepared application is essential for reducing potential review times.
- FDA Workload: The volume of applications and the resources allocated to the FDA can directly affect review speed.
Importantly, the FDA considers the advisory committee's recommendations and other information before making a final decision on what is premarket approval.
Considering these variables, manufacturers are encouraged to prepare for potential delays and allocate sufficient time for each phase of the PMA procedure, as understanding what is premarket approval is essential for ensuring a strong submission that meets FDA expectations. As Katherine Ruiz, a specialist in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, emphasizes, 'Understanding the intricacies of the PMA procedure, including the role of advisory committees, is crucial for navigating the complexities of medical device regulation.
Best Practices for Successful PMA Submissions
To enhance the likelihood of a successful submission, it is essential to understand what is premarket approval and adopt the following best practices:
- Thorough Documentation: Compile all data meticulously and ensure it is well-organized, which significantly facilitates the FDA's evaluation.
- Early Engagement with the FDA: Initiate discussions early to solicit feedback on your submission plan, allowing you to identify and address potential issues proactively. As Chris Rush emphasizes,
I cannot stress the importance of [your clinical data](https://greenlight.guru/blog/strategies-for-successful-pma-submissions-a-guide-for-clinical-teams) (and non-clinical testing data) enough—this highlights the necessity of comprehensive data collection. - Quality Control: Implement stringent quality control measures throughout your studies to maintain the integrity of your data, which is crucial for regulatory approval.
- Stakeholder Collaboration: Cultivate robust communication and collaborative efforts among all stakeholders involved in the project, fostering a united approach to the PMA process.
- Review and Revise: Before submission, have your application examined by experienced professionals, such as Katherine Ruiz, a specialist in Regulatory Affairs for medical products and in vitro diagnostics in Colombia, to identify any possible gaps or weaknesses. Given the recent influx of 471 new life science professionals, it is crucial to recognize the evolving landscape of medical equipment regulation.
Additionally, the 21st Century Cures Act represents a significant legislative change that affects the PMA procedure, making it essential to understand what is premarket approval and its implications. Furthermore, consider the leadership of Dr. Michelle E. Tarver at CDRH, FDA, which emphasizes the significance of diverse representation in medical evaluations. By following these best practices, you can greatly improve the likelihood of a seamless and successful PMA submission, especially as the environment changes with new regulations shaped by initiatives like the 21st Century Cures Act and updates on medical equipment standards.
Leveraging comprehensive clinical trial management services, including trial set-up, monitoring, early-feasibility studies, pivotal studies, and the import permit process for investigational devices, will further support your pathway to approval.
Conclusion
The Premarket Approval (PMA) process is an indispensable framework within the medical device regulatory landscape, particularly for high-risk devices that demand rigorous scrutiny to ensure patient safety. The article has elucidated the various stages of the PMA process, from the initial Pre-Submission phase through to the final decision by the FDA. Each step is critical, requiring meticulous attention to detail and comprehensive documentation to facilitate a smooth review process.
Key differences between the PMA and the 510(k) pathway highlight the necessity for manufacturers to choose the appropriate submission route based on the risk classification of their devices. Understanding these distinctions is essential for developing effective regulatory strategies and ensuring timely market access. Moreover, the article emphasizes the importance of proactive engagement with the FDA and the implementation of best practices in documentation and quality control, which can significantly influence the outcome of PMA submissions.
As the medical device industry continues to evolve, so too do the regulatory requirements and expectations. Stakeholders must remain vigilant in adapting to these changes, leveraging expert guidance and comprehensive clinical trial management services to navigate the complexities of the PMA process. Ultimately, a successful PMA submission not only reflects compliance with regulatory standards but also underscores a commitment to advancing public health through the introduction of safe and effective medical devices.
Frequently Asked Questions
What is premarket approval (PMA)?
Premarket approval (PMA) is a regulatory procedure established by the FDA for assessing specific medical instruments, particularly Class III devices, which are considered higher risk. It evaluates the safety and effectiveness of these products and ensures compliance with regulatory criteria before they can enter the U.S. market.
Why is PMA important for manufacturers?
PMA is crucial for manufacturers as it signifies adherence to regulatory requirements, which is essential for the successful commercialization of medical devices. It involves extensive scientific review to maintain industry standards and safeguard public health.
What types of studies are involved in the PMA process?
The PMA process includes various types of clinical studies, such as Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
What happens if a Class III product does not meet PMA requirements?
Class III products that do not meet PMA requirements are considered adulterated and are prohibited from being marketed, highlighting the critical nature of the PMA approval pathway.
How does engaging with the FDA early benefit the PMA process?
Engaging early with the FDA during the Pre-Submission phase helps clarify expectations and requirements for the product, reducing potential delays during the application process.
What is involved in preparing the PMA application?
Preparing the PMA application requires manufacturers to compile comprehensive data demonstrating the product's safety and effectiveness, along with detailed manufacturing methods and proposed labeling.
What occurs during the FDA review phase of the PMA process?
During the FDA Review phase, the agency meticulously evaluates the submitted application. This may include interactive reviews of the Post-Approval Study (PAS) protocol and convening an advisory committee for external expertise.
What happens after the FDA completes its review of the PMA application?
After thorough evaluation, the FDA will either approve or deny the PMA application. If approved, manufacturers must still conduct post-approval studies to ensure ongoing safety and effectiveness of the high-risk products.
What is the significance of the demographic analysis of FDA panel members?
The demographic analysis of FDA panel members reveals a lack of diversity, with a majority being male and White, indicating a need for a more representative decision-making body within the FDA.
How do companies like bioaccess® support the PMA process?
Companies like bioaccess® provide comprehensive clinical trial management services, ensuring that studies are designed to meet evolving regulatory expectations, thus facilitating a smoother PMA approval experience.




