Introduction
The intricate landscape of medical device design is shaped by a multitude of factors, from engineering principles to regulatory compliance, all aimed at enhancing patient care. As the Medtech industry evolves, particularly in regions like Latin America, companies face distinctive challenges, including navigating complex regulations and limited resources.
This article delves into the fundamental processes involved in medical device development, emphasizing the importance of:
- User-centered design
- Risk management
- Regulatory navigation
By exploring these key elements, it becomes evident how they collectively contribute to the creation of innovative medical solutions that prioritize safety, efficacy, and user satisfaction. With advancements in technology and a growing focus on user experience, the future of medical device design promises to be both dynamic and transformative.
Fundamentals of Medical Device Design
The design of medical devices is a complicated and cross-disciplinary undertaking that combines engineering, user experience, and regulatory adherence. At its foundation, the goal of the design of medical devices is to develop products that not only fulfill specific clinical needs but also prioritize patient safety and treatment efficacy. In Latin America, Medtech companies face unique challenges, including regulatory hurdles, limited financial resources, and the need for robust clinical research frameworks.
In contrast, US Medtech startups grapple with issues such as prolonged subject recruitment timelines and professionalism, which can complicate their efforts in Latin America. Key principles of this development methodology for the design of medical devices involve:
- A profound understanding of the target user group
- Clear identification of clinical requirements
- Strict adherence to regulatory standards, particularly those established by INVIMA, Colombia's National Food and Drug Surveillance Institute, which is acknowledged as a Level 4 health authority by PAHO/WHO.
A robust development process commences with comprehensive research and analysis, which informs the design of medical devices aimed at creating innovative solutions to improve patient care outcomes.
bioaccess® offers a range of services including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
These services are essential for navigating these challenges. As we move towards 2024, recent advancements highlight an increasing emphasis on user experience trends that improve usability and effectiveness, reinforcing the significance of user-focused approaches in attaining optimal clinical outcomes. Notably, the collaboration between Greenlight Guru and bioaccess™ aims to streamline clinical trials and accelerate the market entry of Medtech innovations in Latin America, exemplified by PAVmed's successful first-in-human study in Colombia.
The global market for wearable technology is expected to reach $161 billion by 2033, highlighting the significant growth potential in this sector. Furthermore, challenges like high expenses, restricted accessibility, and negative side effects are essential factors that affect the market for weight loss medications, which in turn can influence the design of medical devices used in bariatric surgery. The case study titled 'Impact of Weight Loss Drugs on Healthcare Equipment Sector' illustrates this interplay, revealing that despite initial concerns about the surge in demand for weight loss medications like Ozempic and Wygovy, analysts believe that high costs and limited accessibility will maintain the necessity for healthcare treatments in the future.
Key Processes in Medical Device Development
The design of medical devices involves several critical processes:
- Concept generation
- Prototyping
- Rigorous testing
- Regulatory approval
At first, a concept arises from recognizing particular clinical requirements, directing the structure and functionality of the apparatus. This is followed by the creation of prototypes, which serve to assess design feasibility and address technical challenges early in the development cycle.
Comprehensive testing, including both preclinical and clinical trials, is paramount to evaluate the safety and effectiveness of the product in real-world scenarios. In this context, clinical trial management services are essential, offering:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Detailed reporting on study status, inventory, and both serious and non-serious adverse events
Significantly, INVIMA, the Colombia National Food and Drug Surveillance Institute, serves an essential function as the regulatory body supervising these activities, categorized as a Level 4 health authority by PAHO/WHO, ensuring safety, efficacy, and quality in healthcare products.
In 2024, the labor productivity in the Medical Devices & Products market is projected to reach US$159.0k, indicating a robust and growing sector. Furthermore, advancements in 3D printing technology are revolutionizing the industry, as it is more cost-effective and can reduce energy consumption from manufacturing, storing, and shipping parts by up to 64%. The market for 3D printed healthcare products is anticipated to attain $6.9 billion by 2028, highlighting the growing investment and significance of this technology.
Additionally, the FDA now mandates that cybersecurity be integrated into the review process for products containing software, reflecting the increasing reliance on smart, connected medical equipment. A relevant case study emphasizes that with the rise of these products, manufacturers must adhere to updated cybersecurity guidance, ensuring compliance with safety and quality regulations while maintaining transparency in their cybersecurity controls. According to industry analysts, with the right managerial strategies and high flexibility, companies can succeed and strengthen their long-term market position.
Adhering to regulatory requirements is essential, as it guarantees that products meet established standards of safety and efficacy before they can be introduced to the market. Katherine Ruiz, a Regulatory Affairs expert, has significantly contributed to this field, advising foreign manufacturers on obtaining market clearance for their innovations in Colombia, leveraging her extensive background from INVIMA to navigate the complexities of regulatory compliance.
User-Centered Design in Medical Devices
User-focused methodology (UCD) is an essential approach that prioritizes the user in the medical equipment development process. This involves actively engaging end-users—comprising both healthcare professionals and patients—through interviews, surveys, and extensive usability testing. Such approaches are essential for revealing users' needs, preferences, and challenges, which in turn guide the design of medical devices that are suited to their real-world usage.
Annette DeVito Dabbs, RN, PhD, emphasizes the importance of this method, stating,
Involving patient-users in the creation and testing ensured functionality and usability, therefore increasing the likelihood of promoting the intended health outcomes.
This perspective is increasingly relevant as the industry pivots towards models that embrace user feedback and iterative design processes in the design of medical devices. For instance, the development of the handheld gadget for Pocket PATH illustrates the practical application of UCD; the team selected the Hewlett Packard iPAQ Pocket PC to enable lung transplant patients to self-monitor at home, addressing geographical challenges while promoting ease of use and integration into daily routines.
Additionally, the latest courses on Design and Development Plan and Risk Management Plan, priced at €49 and taking 1-2 hours to complete, align with ISO 13485 and ISO 14971:2019 standards, emphasizing the critical nature of regulatory compliance alongside user-centered methodologies. As we near 2024, statistics show an increasing acknowledgment of UCD's importance in healthcare tools, with a substantial effort towards gathering user feedback to improve safety and effectiveness. Furthermore, Lewis Jr's evaluation of IBM computer usability satisfaction questionnaires underscores the importance of usability testing in this context.
This trend not only encourages innovation but also ultimately results in tools that significantly enhance patient outcomes.
Navigating Regulatory Requirements in Medical Device Design
Navigating the regulatory landscape is essential for the successful design and commercialization of medical devices. In the United States, the Food and Drug Administration (FDA) is the authoritative body overseeing the approval procedure, mandating that manufacturers submit comprehensive documentation demonstrating the safety and efficacy of their products. Our comprehensive clinical trial management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting on study status
- Feedback on study documents to ensure adherence to country requirements
This meticulous management covers all aspects of the clinical trial.
As we look ahead to 2024, FDA approval statistics indicate a noteworthy shift, with panel track wait times increasing from 285.80 days in 2023 to 289.62 days—a 1.3% rise. This trend, while presenting challenges, is viewed positively by analysts. Iseult McMahon, an analyst at BTIG, notes,
We think that this is a directional positive for the broader medical technology space as it provides both improving certainty to companies (and investors) as well as reduces cash burn during the interim period when a company is awaiting a decision.
The increase in wait times can strain manufacturers' resources and impact their strategic planning, making it vital for them to adapt their operational strategies accordingly. For example, Artivion recently encountered substantial operational interruptions due to a ransomware attack that impacted its shipping activities. This incident underscores the importance of robust compliance and risk management strategies in navigating regulatory challenges.
Internationally, compliance with standards such as ISO 13485 and the Medical Device Regulation (MDR) in Europe introduces additional requirements that manufacturers must adhere to. For example, companies must implement quality management systems that not only meet regulatory expectations but also enhance product reliability and safety. Comprehending these regulations at the beginning of the development process is vital; it not only guarantees adherence but also reduces the risk of expensive delays and fosters trust among stakeholders.
With recent changes in international healthcare equipment regulations, the focus on quality management systems and compliance with established standards is more crucial than ever for technology firms aiming to succeed in a competitive landscape. Our team, including experts like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, a Regulatory Affairs expert, is equipped to guide you through these complexities, ensuring your clinical trials are successful and compliant.
Risk Management in Medical Device Design
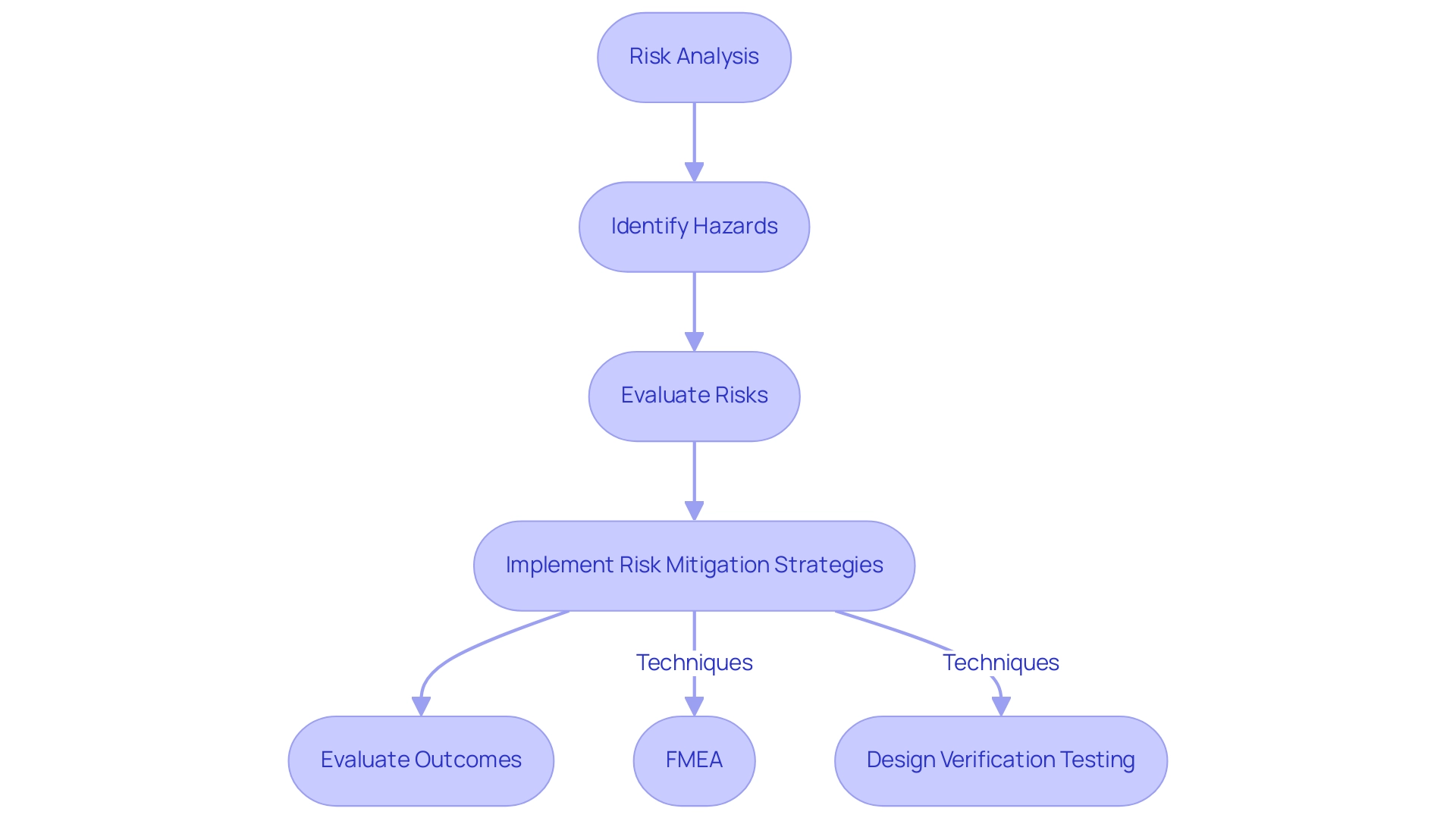
Risk management acts as a fundamental aspect in the design of medical devices, concentrating on the identification, evaluation, and reduction of potential hazards linked to their use. This intricate process commences with a thorough risk analysis to evaluate possible hazards that may arise during the product's lifecycle. Subsequently, manufacturers implement robust strategies to minimize these risks.
Among the widely recognized techniques employed in the design of medical devices are:
- Failure Mode and Effects Analysis (FMEA)
- Design verification testing
Both of which play critical roles in ensuring that potential failures are systematically identified and addressed. FMEA helps teams prioritize potential failure modes based on their severity, occurrence, and detection, allowing for targeted risk mitigation efforts.
By prioritizing these risk management practices, manufacturers not only enhance the safety and efficacy of their devices but also significantly contribute to improved patient outcomes. The latest developments in risk management are continuously evolving, reflecting the industry’s commitment to adopting best practices that uphold the highest safety standards.
Additionally, interested users can book a free custom demo to explore Ultralight's services, which may provide further insights into effective risk management strategies and tools available in the market.
Conclusion
The multifaceted nature of medical device design underscores the critical importance of integrating user-centered design, effective risk management, and navigating regulatory landscapes. Each of these elements plays a vital role in ensuring that medical devices are not only innovative but also safe and effective for end-users. By prioritizing user engagement throughout the design process, manufacturers can create solutions that truly address the needs of healthcare providers and patients alike, ultimately leading to better health outcomes.
As the Medtech industry continues to evolve, particularly in regions like Latin America, the challenges faced by companies must be met with robust strategies and an unwavering commitment to compliance and safety. The insights provided into the regulatory environment highlight the necessity for manufacturers to stay informed and adaptable, ensuring their products meet the rigorous standards set by authorities such as INVIMA and the FDA.
Looking ahead, advancements in technology, such as 3D printing and the integration of cybersecurity measures, further enhance the potential for innovation within the sector. However, it remains imperative for companies to maintain a strong focus on risk management practices to mitigate potential hazards throughout the device lifecycle. By embracing these principles, the Medtech industry can continue to push the boundaries of what is possible, driving forward the development of medical devices that not only meet regulatory requirements but also enhance patient care and satisfaction.
Frequently Asked Questions
What is the primary goal of designing medical devices?
The primary goal of designing medical devices is to develop products that fulfill specific clinical needs while prioritizing patient safety and treatment efficacy.
What challenges do Medtech companies face in Latin America?
Medtech companies in Latin America face challenges such as regulatory hurdles, limited financial resources, and the need for robust clinical research frameworks.
What are some key principles in the development methodology for medical devices?
Key principles include a profound understanding of the target user group, clear identification of clinical requirements, and strict adherence to regulatory standards.
What services does bioaccess® offer to assist in the development of medical devices?
Bioaccess® offers services including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
How is user experience being emphasized in medical device design?
There is an increasing emphasis on user experience trends that improve usability and effectiveness, reinforcing user-focused approaches to achieve optimal clinical outcomes.
What critical processes are involved in the design of medical devices?
The critical processes include concept generation, prototyping, rigorous testing, and regulatory approval.
What role does INVIMA play in the regulation of medical devices in Colombia?
INVIMA, Colombia's National Food and Drug Surveillance Institute, serves as the regulatory body overseeing the safety, efficacy, and quality of healthcare products.
How is 3D printing technology impacting the medical device industry?
Advancements in 3D printing technology are revolutionizing the industry by being more cost-effective and reducing energy consumption, with the market for 3D printed healthcare products projected to reach $6.9 billion by 2028.
What is the importance of user-centered design (UCD) in medical device development?
UCD prioritizes the user by engaging healthcare professionals and patients in the development process, ensuring that devices meet real-world needs and enhance patient outcomes.
What are the key aspects of navigating the regulatory landscape for medical devices?
Key aspects include compliance with FDA regulations, submission of comprehensive documentation, and adherence to international standards such as ISO 13485.
What is the significance of risk management in the design of medical devices?
Risk management is crucial for identifying, evaluating, and reducing potential hazards associated with medical devices, ensuring their safety and efficacy throughout their lifecycle.
What techniques are commonly used in risk management for medical devices?
Common techniques include Failure Mode and Effects Analysis (FMEA) and design verification testing, which help prioritize and address potential failures systematically.




