Overview
Usability engineering in medical devices is a structured approach focused on designing and assessing tools to enhance their effectiveness for end-users, thereby improving user satisfaction and patient safety. The article outlines how methodologies, regulatory standards like IEC 62366, and the incorporation of human factors are essential in creating intuitive devices that minimize errors and align with user needs, ultimately leading to better health outcomes.
Introduction
The integration of usability engineering in medical devices is increasingly recognized as a pivotal factor in enhancing user experience and ensuring patient safety. This structured methodology not only focuses on the design and evaluation of devices to maximize usability for healthcare professionals and patients but also addresses the complex interplay between user needs and regulatory requirements.
As the landscape of medical technology evolves, manufacturers are called to prioritize user-centered design principles, leading to innovations that improve health outcomes and reduce errors.
With the growing emphasis on regulatory compliance and the incorporation of human factors, the usability engineering process has become a cornerstone for developing effective medical devices.
This article delves into the key components of usability engineering, explores the regulatory frameworks that govern it, and highlights the future trends that are set to shape this essential discipline in healthcare technology.
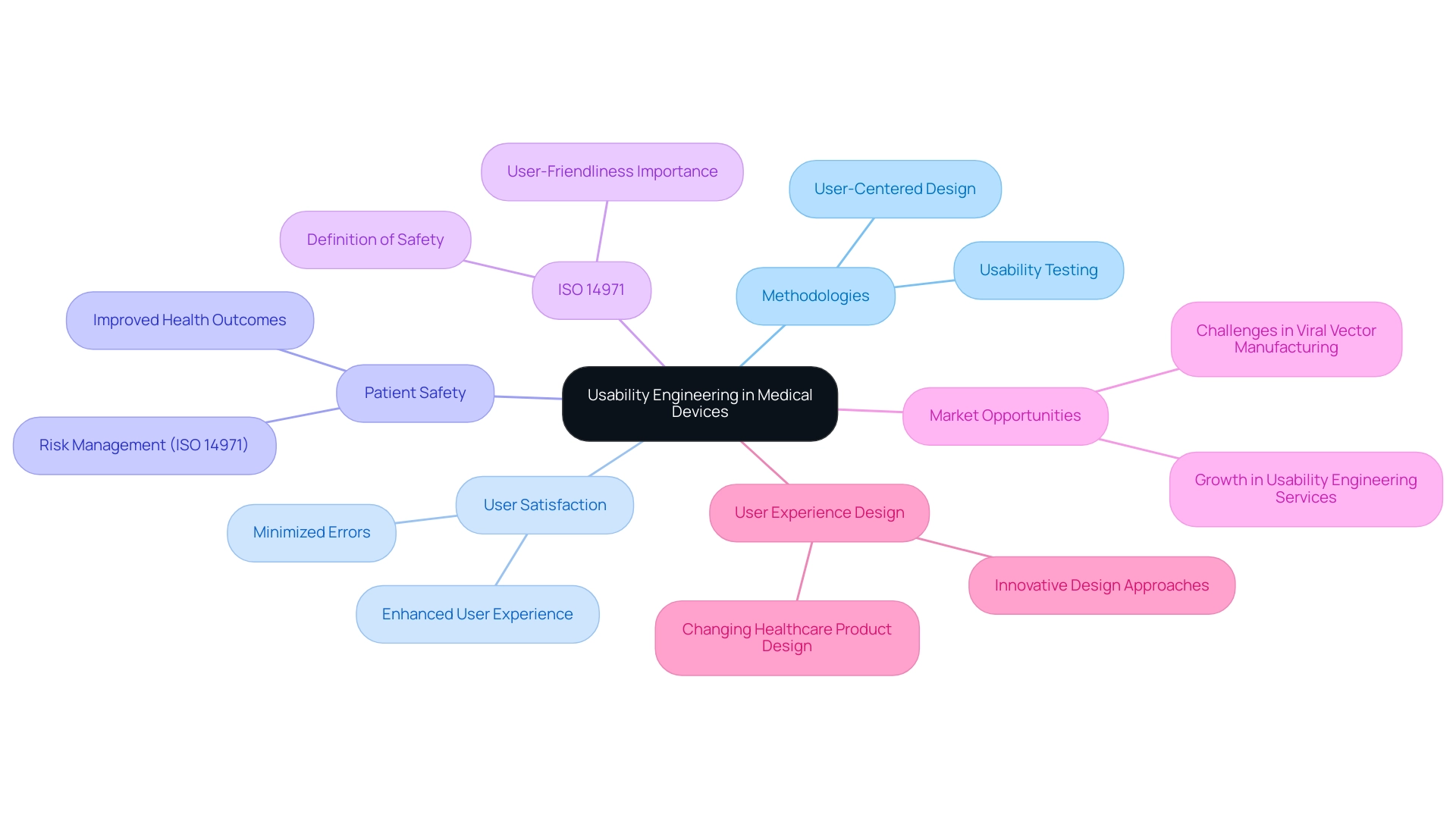
Defining Usability Engineering in Medical Devices
Usability engineering medical devices is a structured approach aimed at designing and assessing tools to enhance their effectiveness for end-users, including healthcare professionals and patients. This approach includes various established methodologies from usability engineering medical devices that ensure tools are not only intuitive and easy to use but also customized to meet specific requirements. By focusing on usability engineering medical devices, manufacturers can significantly enhance user satisfaction, minimize the potential for errors, and ultimately improve patient safety and health outcomes.
As Michaela Kauer-Franz, a recognized authority in the medical device sector, states,
ISO 14971, the risk management standard, defines safety as 'freedom from unacceptable risk', emphasizing that user-friendliness is essential to attaining safety in healthcare technology. Furthermore, the report forecasts considerable opportunities within the usability engineering medical devices and design engineering services sector, particularly as the demand for user-centered design continues to expand. For those seeking to improve their comprehension of user experience engineering, an online course is available that instructs on how to create a pragmatic user experience engineering process in just 8 hours.
Furthermore, the restricted market for commercial production of viral vectors, as demonstrated by the case study showing that most of the 24 companies are CDMOs, highlights the specialized challenges within engineering for use. Beyond simple aesthetics, usability engineering medical devices requires a deep comprehension of interactions and the situations in which tools are utilized, establishing it as a crucial element throughout the lifecycle of healthcare instruments. This emphasis on functionality is becoming more significant, particularly as the field of healthcare product user experience design keeps changing, offering chances for innovation and enhanced care delivery.
Regulatory Frameworks and Standards for Usability Engineering
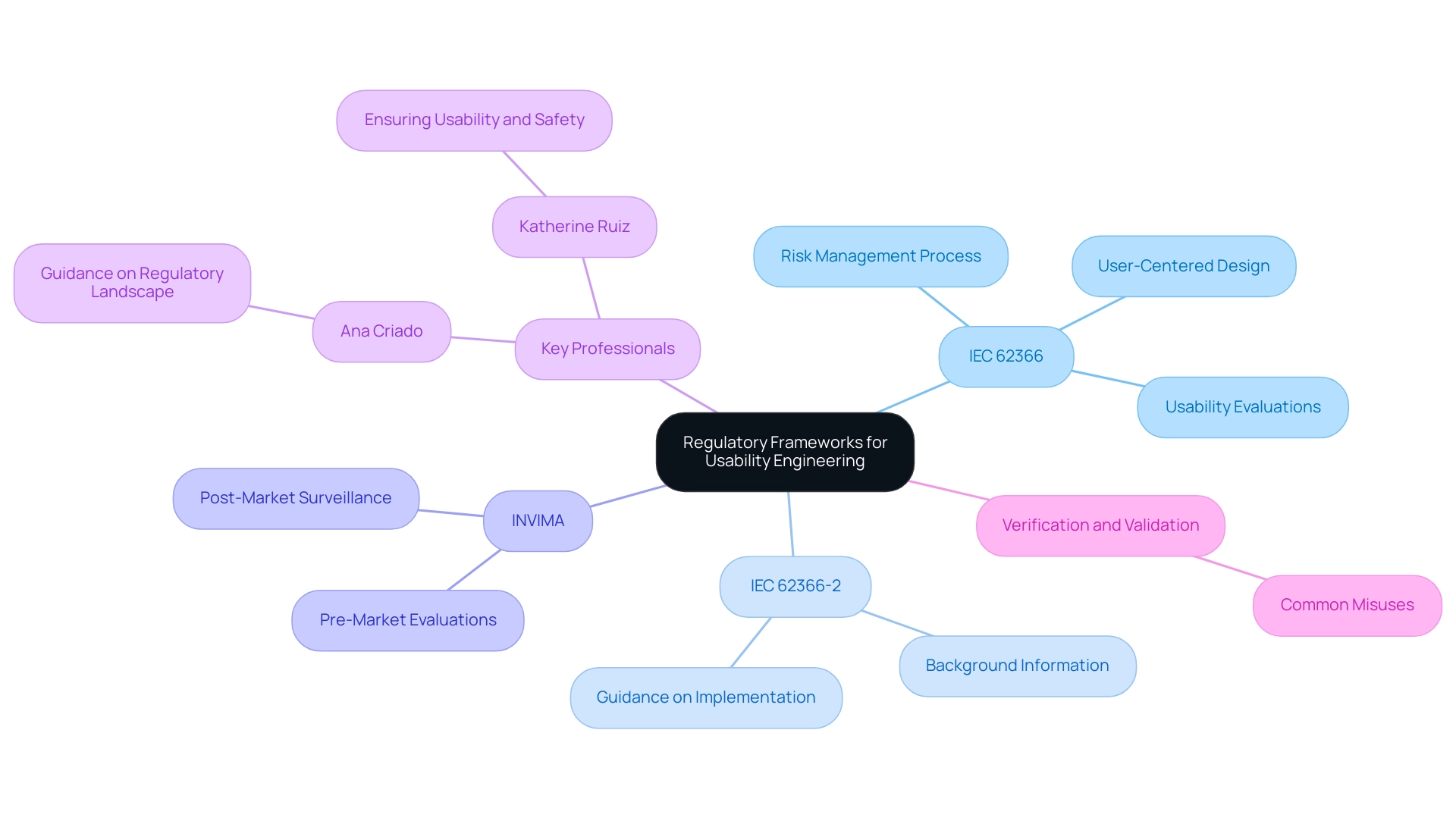
The process of usability engineering medical devices for healthcare tools is intricately regulated by various frameworks and standards designed to ensure both safety and effectiveness. A significant standard in this domain is IEC 62366, which outlines comprehensive guidelines for incorporating user-centered design into the lifecycle of medical devices. Additionally, IEC 62366-2 serves as a technical report that offers essential background information and guidance on implementing IEC 62366-1, further improving the engineering process.
This standard emphasizes the necessity of a robust risk management process specifically targeting usability concerns in usability engineering medical devices, necessitating manufacturers to conduct usability evaluations at various stages of product development. Such evaluations are not merely bureaucratic hurdles; they play a critical role in identifying and mitigating potential risks associated with usage. Adherence to IEC 62366, combined with a comprehension of the proper application of verification and validation terminology, strengthens the safety profile of health products and improves manufacturers' credibility with regulatory authorities and consumers.
In Colombia, the National Food and Drug Surveillance Institute (INVIMA) plays a pivotal role in this landscape, acting as a Level 4 health authority recognized by PAHO/WHO, tasked with overseeing the marketing and safety of health products. INVIMA employs specific methodologies, including pre-market evaluations and post-market surveillance, to ensure compliance with safety standards. Professionals such as Ana Criado, Director of Regulatory Affairs and a specialist in biomedical engineering, and Katherine Ruiz, an expert on regulatory matters for healthcare products, contribute significantly to ensuring adherence to these standards.
- Ana Criado, with her extensive experience at INVIMA, plays a crucial role in guiding companies through the regulatory landscape, while
- Katherine Ruiz focuses on ensuring that healthcare products meet the necessary usability and safety requirements.
As Vladimir Terekhov points out, 'The journey from concept to market for healthcare software is intricate, requiring a deep understanding of both technical and regulatory aspects.' The landscape of healthcare product software development is continuously evolving, with manufacturers facing complex regulatory requirements while striving for innovation.
Following these standards ultimately aids in the development of safer, more effective products through usability engineering medical devices, thereby enhancing patient care and progressing the field of technology.
The Role of Human Factors in Usability Engineering
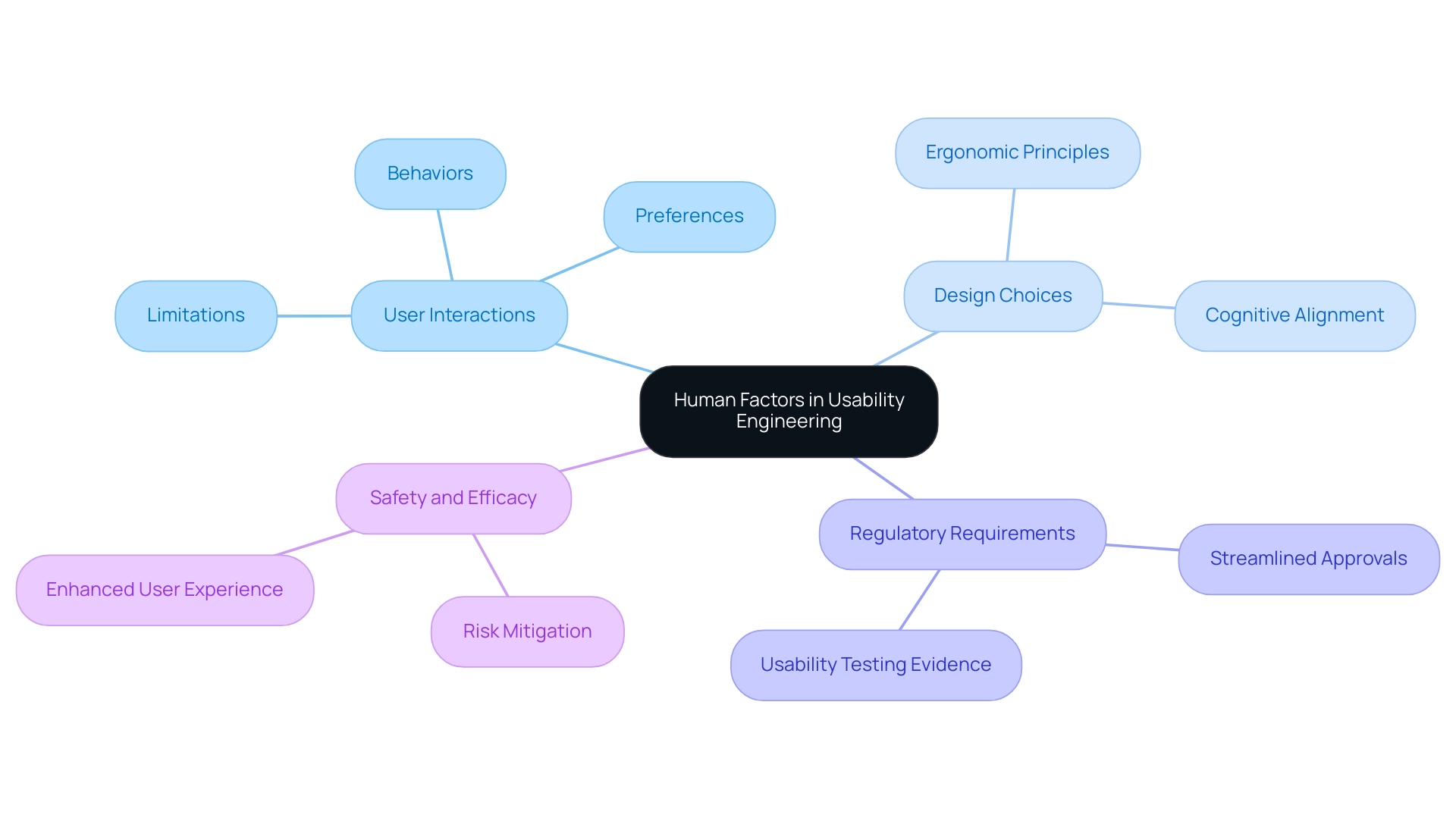
Human elements are essential to interface engineering, concentrating on the complex ways individuals interact with medical devices. This discipline involves a comprehensive analysis of individual behaviors, preferences, and inherent limitations, which directly informs design choices. Regulatory bodies require evidence of user testing that considers human factors to streamline approvals, thereby accelerating time-to-market.
By incorporating human factors into usability engineering medical devices, manufacturers can create products that are not only operationally effective but also aligned with the cognitive and physical abilities of their users. Such a user-centered approach significantly mitigates the risks of misuse and errors, enhancing overall safety and efficacy. For example, ergonomic design principles—highlighted in recent studies—can substantially enhance comfort and efficiency during usage.
As Melanie Camp noted in the Journal of Radiology Nursing (Volume 37, Issue 2, 2018, pp. 77-84), effective integration of human factors in design is crucial for fostering safer healthcare environments. Additionally, the case study titled "Regulatory Framework for Alarm Guidelines" highlights the absence of thorough regulatory supervision in medical equipment alarms, emphasizing the importance of prioritizing human elements in engineering for user-friendliness.
Hence, it is imperative for manufacturers to prioritize usability engineering medical devices and human factors throughout the development process, ensuring that devices meet the nuanced needs of healthcare professionals and patients alike.
Key Activities in the Usability Engineering Process
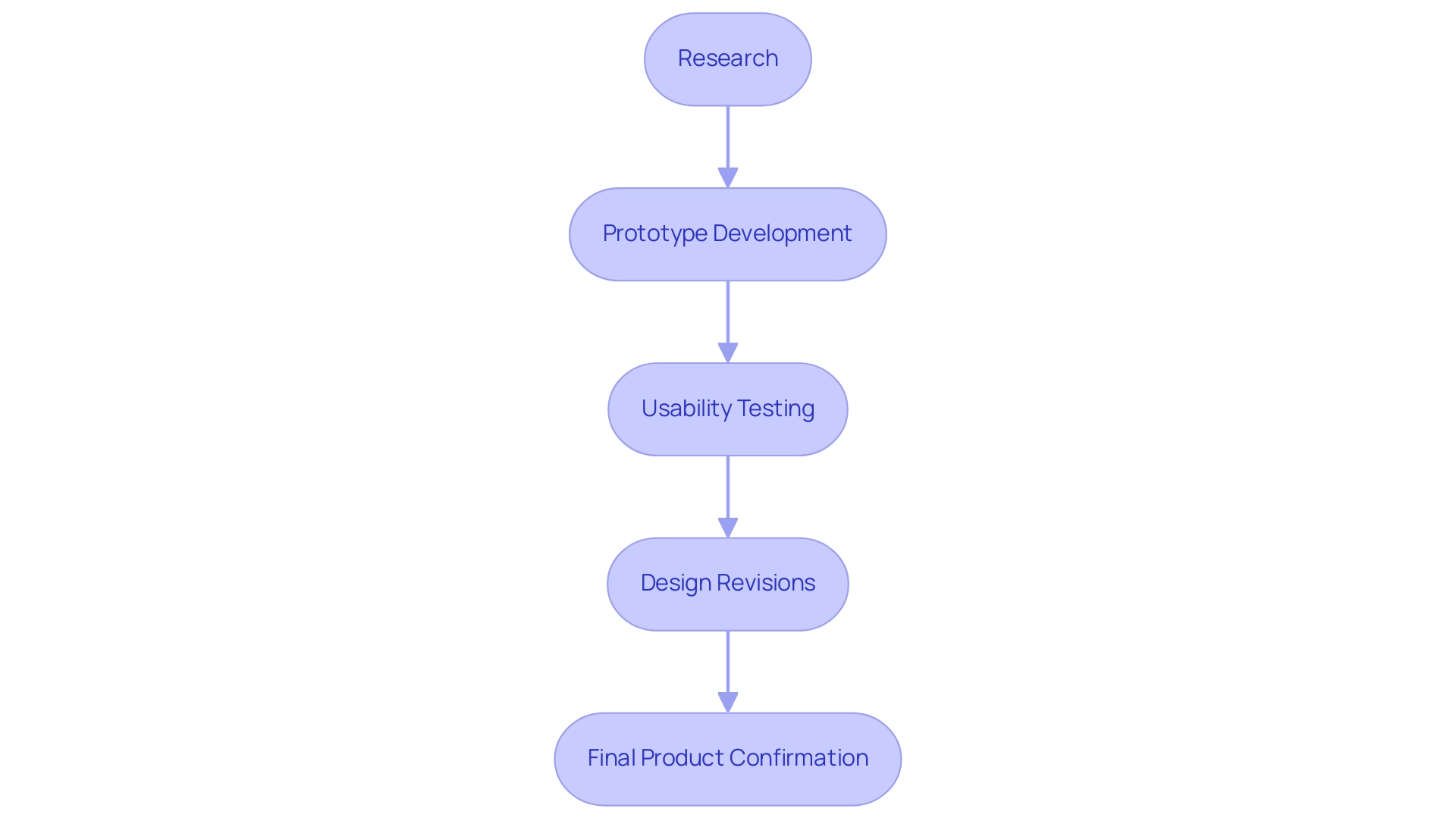
The usability engineering medical devices process includes several essential activities, such as research, usability testing, and iterative design. At first, research is essential for gathering insights into the needs and expectations of potential individuals. This foundational step informs the design process by ensuring that requirements from individuals are prioritized.
Following this, prototypes are developed and subjected to rigorous usability testing, which is vital for uncovering usability issues. To achieve reliable results, practitioners must consider the statistic that to detect problems affecting at least 5% of individuals with 95% confidence, around 59 participants are needed. Various measurements are employed during this testing phase, including:
- Tracking the number of errors
- Repeated mistakes
- The time taken to complete tasks
- Incorrect menu selections
Input gathered from experience testing influences design revisions, ensuring that the final product aligns closely with expectations. Usability engineering medical devices emphasizes ease of use and effectiveness through research and testing, while interaction design focuses on creating interactive elements within a product. Both formative and summative assessments play a crucial role in confirming the effectiveness of the product prior to market launch.
For instance, Klickkonzept, a German performance agency, significantly enhanced experience, resulting in a remarkable 19% increase in revenue. By executing these essential tasks, manufacturers can significantly improve the usability engineering of medical devices, resulting in enhanced user experiences and superior health outcomes. As emphasized by UX Designer Adrianna Modrzyńska, the field thrives on 'blending creativity and strategic thinking to engineer captivating user experiences that redefine the digital landscape.
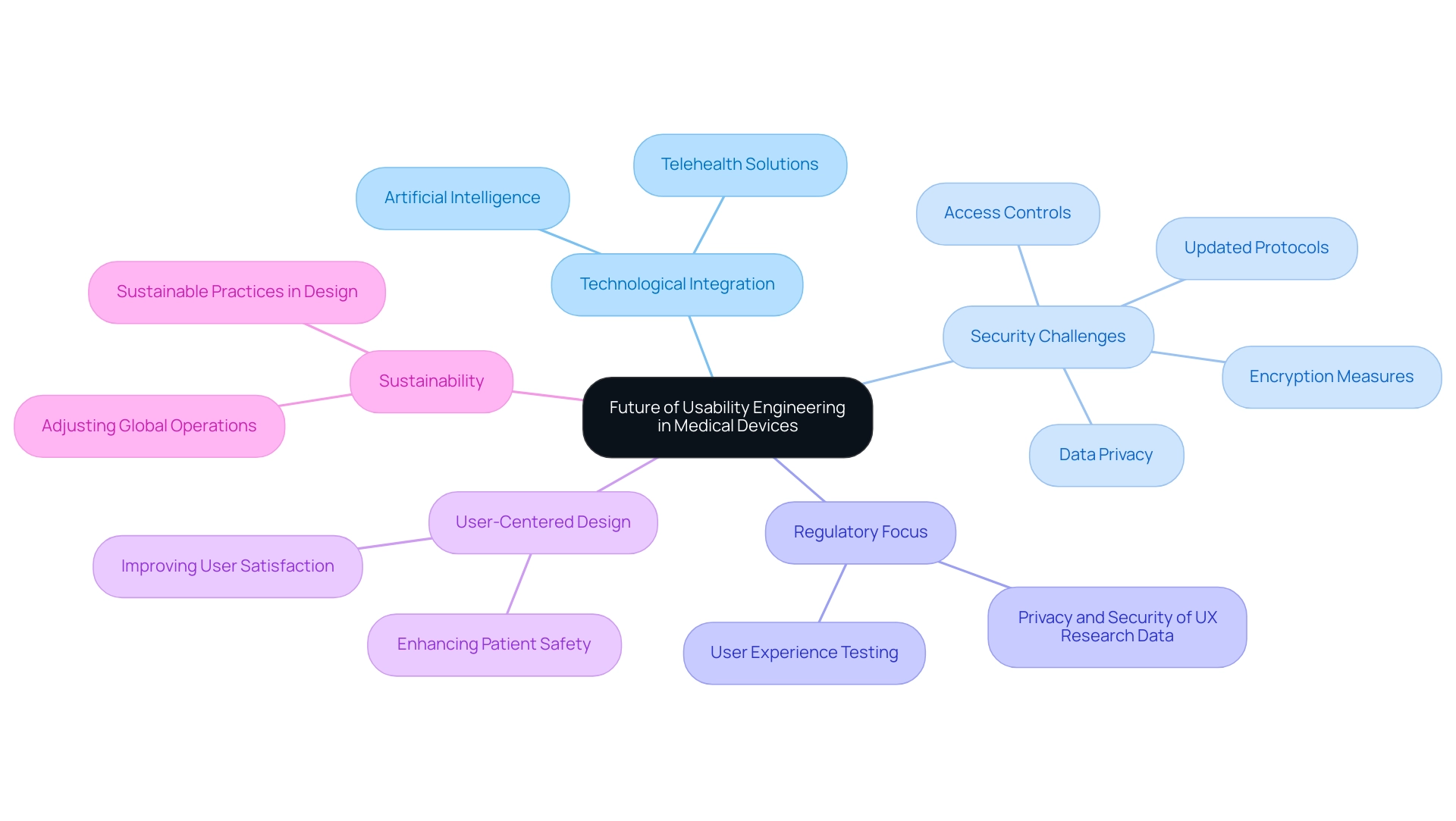
The Future of Usability Engineering in Medical Devices
The future of usability engineering medical devices in healthcare tools is poised for significant change, driven by swift technological progress and an increased emphasis on user-centered design. As the healthcare landscape evolves, there is an increasing acknowledgment that usability engineering medical devices is crucial for enhancing both patient safety and satisfaction. Notably, trends such as the integration of artificial intelligence and telehealth solutions are reshaping how individuals interact with medical devices, thereby prompting a necessary reevaluation of usability engineering medical devices.
In this context, the challenge of achieving optimal network security while satisfying expectations becomes paramount.
You're striving for optimal network security. How can you meet client expectations while safeguarding data?
Tackling this challenge is crucial, as strong security measures can improve experience by building trust and making individuals feel secure while engaging with healthcare equipment. Furthermore, regulatory bodies are expected to intensify their focus on usability engineering medical devices by enhancing user experience testing during the approval process, making it vital for manufacturers to prioritize the privacy and security of UX research data through:
- Encryption
- Access controls
- Updated protocols
By proactively adopting innovative usability engineering medical devices practices that prioritize user needs and experiences, manufacturers can ensure that health-related tools remain effective and safe in a continuously evolving healthcare environment.
As nearly 80% of medical device companies plan to adjust their global operations or supply chains to address sustainability concerns, it is crucial to understand how this shift affects engineering for user experience. Aligning usability engineering medical devices with these broader market shifts will be critical for future success, as manufacturers will need to consider sustainable practices in their design and development processes.
Conclusion
The integration of usability engineering in medical devices is not merely a trend but a fundamental necessity for enhancing user experience and ensuring patient safety. By employing structured methodologies that prioritize user-centered design, manufacturers can significantly improve the usability of medical devices, thereby minimizing errors and fostering better health outcomes. The emphasis on usability engineering is further underscored by the critical regulatory frameworks and standards, such as IEC 62366, which guide manufacturers in embedding usability considerations throughout the device lifecycle.
Human factors play a pivotal role in this domain, as understanding user behaviors and preferences is essential for creating devices that are intuitive and effective. By conducting thorough user research and rigorous usability testing, manufacturers can identify potential issues early in the development process, leading to iterative improvements that align closely with user needs. This proactive approach not only enhances the safety and efficacy of medical devices but also accelerates the time to market, ensuring that innovations reach healthcare professionals and patients swiftly.
Looking ahead, the future of usability engineering is poised for transformation, particularly with the rise of artificial intelligence and telehealth solutions. These advancements necessitate a continuous reevaluation of usability practices to meet evolving user expectations while maintaining robust security measures. As the landscape of medical technology progresses, manufacturers must adapt to these changes by aligning usability engineering with broader market shifts, including sustainability initiatives. Ultimately, prioritizing usability and human factors will be crucial for developing safe, effective, and user-friendly medical devices that contribute to improved healthcare delivery and patient satisfaction.
Frequently Asked Questions
What is usability engineering in the context of medical devices?
Usability engineering for medical devices is a structured approach aimed at designing and assessing tools to enhance their effectiveness for end-users, including healthcare professionals and patients. It focuses on creating tools that are intuitive, easy to use, and customized to meet specific requirements.
How does usability engineering improve patient safety and health outcomes?
By focusing on usability engineering, manufacturers can enhance user satisfaction, minimize the potential for errors, and ultimately improve patient safety and health outcomes.
What is the significance of ISO 14971 in usability engineering?
ISO 14971, the risk management standard, defines safety as 'freedom from unacceptable risk' and emphasizes that user-friendliness is essential for achieving safety in healthcare technology.
What opportunities exist within the usability engineering medical devices sector?
There are considerable opportunities for growth in the usability engineering medical devices and design engineering services sector, particularly as the demand for user-centered design continues to expand.
What role do standards like IEC 62366 play in usability engineering?
IEC 62366 outlines guidelines for incorporating user-centered design into the lifecycle of medical devices and emphasizes the need for a robust risk management process targeting usability concerns.
What is the purpose of usability evaluations in product development?
Usability evaluations are conducted at various stages of product development to identify and mitigate potential risks associated with usage, thereby enhancing the safety profile of health products.
Who oversees the marketing and safety of health products in Colombia?
The National Food and Drug Surveillance Institute (INVIMA) oversees the marketing and safety of health products in Colombia, employing methodologies such as pre-market evaluations and post-market surveillance.
What are the roles of professionals like Ana Criado and Katherine Ruiz at INVIMA?
Ana Criado guides companies through the regulatory landscape, while Katherine Ruiz ensures that healthcare products meet necessary usability and safety requirements.
How does the regulatory landscape affect healthcare software development?
The journey from concept to market for healthcare software is complex, requiring a deep understanding of both technical and regulatory aspects, as manufacturers face intricate regulatory requirements while striving for innovation.
What is the overall benefit of following usability engineering standards?
Adhering to usability engineering standards ultimately aids in the development of safer, more effective products, enhancing patient care and advancing the field of technology.

