Overview
Validation in clinical data management is a critical process that ensures the accuracy, completeness, and consistency of data collected during clinical trials, which is essential for maintaining the integrity of research outcomes. The article emphasizes that rigorous validation practices, supported by modern technologies and adherence to regulatory standards, significantly enhance data quality and reliability, thereby facilitating informed decision-making and improving patient safety in clinical research.
Introduction
In the intricate world of clinical research, the integrity of data is paramount. As clinical trials become increasingly complex, the necessity for rigorous data validation processes has never been more critical. This article delves into the multifaceted aspects of clinical data validation, highlighting essential definitions, best practices, and the regulatory landscape that governs this vital area.
From the adoption of advanced technologies that streamline validation efforts to the imperative of maintaining compliance with stringent regulatory standards, the discussion provides a comprehensive overview of how these elements converge to ensure the reliability and accuracy of clinical trial outcomes.
By exploring contemporary challenges and innovative solutions, this examination underscores the pivotal role of data validation in enhancing the credibility of research findings and ultimately safeguarding patient welfare.
Understanding Clinical Data Validation: Key Definitions and Concepts
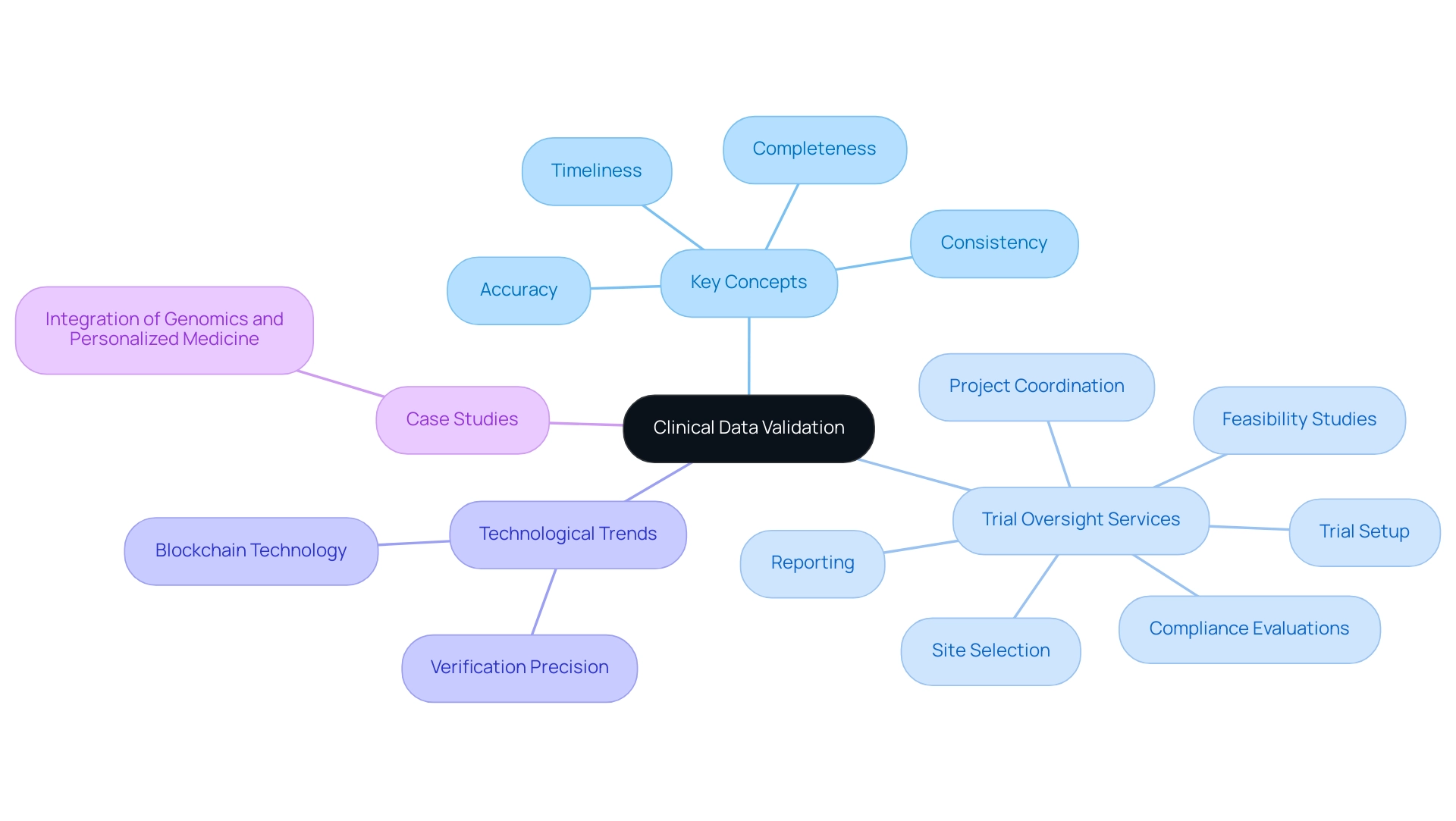
The validation in clinical data management of trial information is a vital procedure focused on guaranteeing the precision, completeness, and consistency of information collected during studies. This process involves a strong series of checks and balances throughout the research lifecycle, which are essential for confirming integrity and adherence to regulatory standards. Key concepts inherent in validation in clinical data management include:
- Accuracy
- Completeness
- Consistency
- Timeliness
These elements are vital for upholding the credibility of research outcomes.
Our thorough trial oversight services include:
- Feasibility studies
- Site selection
- Compliance evaluations
- Trial setup
- Project coordination
- Reporting
This ensures all aspects of the trial are meticulously handled. Specifically, our reporting process includes detailed communication of study status, inventory management, and the documentation of serious and non-serious adverse events, which are critical for maintaining transparency and compliance. Significantly, SaS is extensively employed for its strong abilities in analysis, validation, and decision support, which are essential in improving verification processes.
A recent statistic indicates that 2024 will see a significant emphasis on verification precision in healthcare information, with advancements in methodologies contributing to heightened standards. Moreover, as 60% of pharmaceutical firms are currently investigating blockchain technology to enhance information security, the environment of clinical research information oversight is changing swiftly, highlighting the necessity for creative solutions in this field. Improved methods in validation in clinical data management can assist in spotting mistakes early in the collection process, thus reducing the risk of compromised test outcomes.
Additionally, the role of INVIMA, as a Level 4 health authority by PAHO/WHO, is pivotal in overseeing medical device regulation in Colombia, ensuring compliance with national standards and facilitating the import permit and nationalization of investigational devices. The combination of genomics and personalized medicine, as demonstrated by the case study on 'Integration of Genomics and Personalized Medicine,' exemplifies the transformative potential of precise information management, enabling researchers to analyze genetic markers linked to diseases, resulting in more effective and targeted therapies. Considering these developments, the significance of preserving information integrity in research trials cannot be overstated, as it directly influences the success and reliability of therapeutic interventions.
The Clinical Data Validation Process: Steps and Best Practices
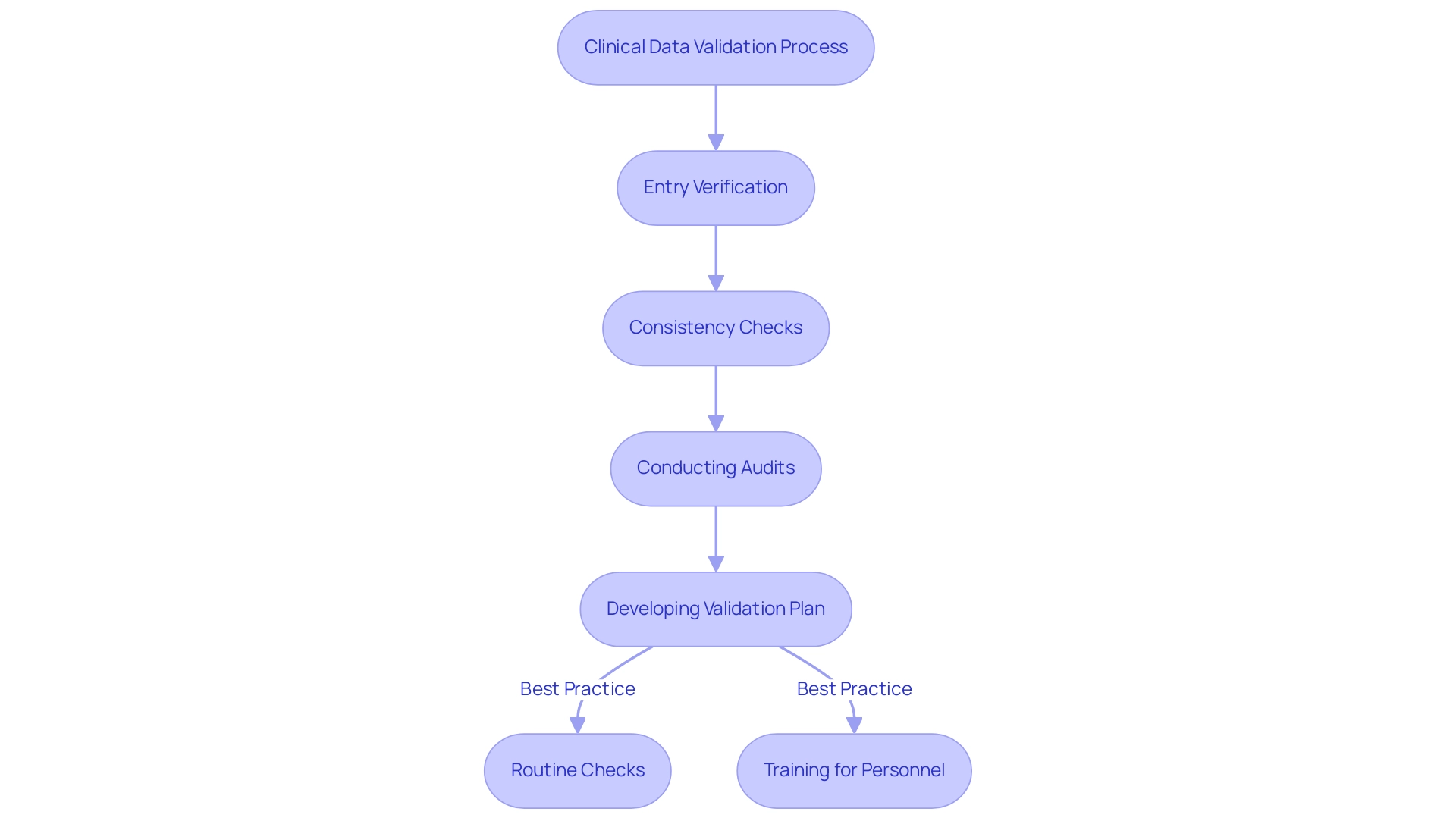
The process of validation in clinical data management encompasses several critical steps designed to ensure the integrity and reliability of collected information. Essential elements consist of entry verification, which acts as the first line of defense against inaccuracies, followed by consistency checks that identify discrepancies within the dataset. Furthermore, conducting thorough audits is essential to validate the overall quality of the information.
Adopting best practices is paramount; for instance, developing a comprehensive validation plan that delineates specific procedures and acceptance criteria can significantly enhance the process. This plan should incorporate routine checks throughout information collection, ensuring that any anomalies are addressed promptly. The incorporation of automated systems, propelled by new technologies like artificial intelligence and blockchain, has transformed information precision in research trials, enhancing the validation procedure while reducing human mistakes.
Consistent training for personnel on revised information handling protocols is similarly essential, promoting a culture of adherence and alertness. According to research specialist Lucy Parker, "A commitment to excellence in information oversight is essential for attaining trustworthy results in medical research." By adhering to these best practices and leveraging advancements in information entry verification techniques, researchers can markedly enhance quality, ultimately leading to more successful trial outcomes.
Notably, a case study on continuous improvement in information management emphasizes how ongoing review and refinement of practices ensure that methodologies remain current and effective, thereby reinforcing the importance of validation in clinical data management. Moreover, implementing Good Data Management Practices (GCDMP) requires a commitment that provides substantial benefits for research, making it an essential focus for all stakeholders.
Regulatory Compliance in Clinical Data Validation: Navigating Guidelines and Standards
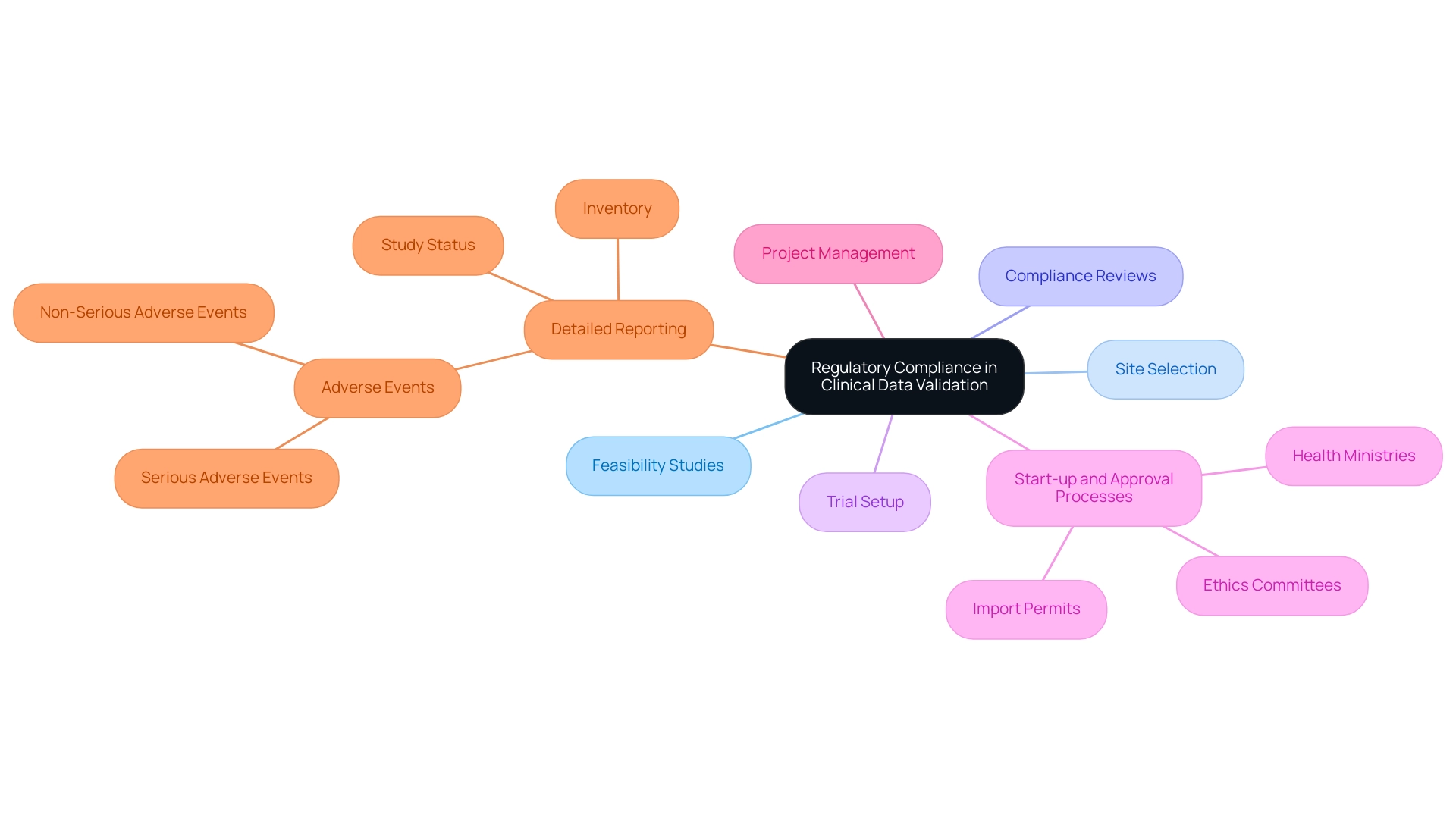
Validation in clinical data management is essential for ensuring regulatory adherence in research and preserving the integrity of studies while ensuring participant safety. Our comprehensive clinical trial management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Start-up and approval processes involving:
- Ethics committees
- Health ministries
- Import permits
- Project management
- Detailed reporting on:
- Study status
- Inventory
- Both serious and non-serious adverse events
These services are all aimed at facilitating adherence to guidelines established by authorities such as the FDA and EMA.
These regulations outline the standards for information integrity, which includes the validation in clinical data management as well as adherence to Good Clinical Practice (GCP) and Good Laboratory Practice (GLP). Navigating these complex regulations can pose significant challenges; for instance, recent information indicates that 27% of security and IT professionals identify mitigating internal audit fatigue from recurring assessments as a leading challenge in compliance programs. Furthermore, 35% of business and tech leaders consider third-party breaches to be one of the most alarming cyber threats, emphasizing the wider implications of compliance and security in data management.
Furthermore, three out of five corporate risk and compliance professionals express confidence in managing compliance risks, yet they also report obstacles such as a lack of knowledgeable personnel and insufficient resources. This highlights the necessity for medical researchers to remain vigilant and informed about evolving regulatory standards to ensure proper validation in clinical data management. With Katherine Ruiz’s expertise in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, we ensure that compliance protects not only the rights and welfare of participants but also enhances the reliability and credibility of research findings.
As emphasized in Inspectorio's State of Supply Chain Report 2024, 40% of supply chain experts prioritize risk oversight and compliance pressures, demonstrating a wider industry acknowledgment of the significance of regulatory conformity in research studies.
Modern Techniques in Clinical Data Validation: Leveraging Technology for Accuracy
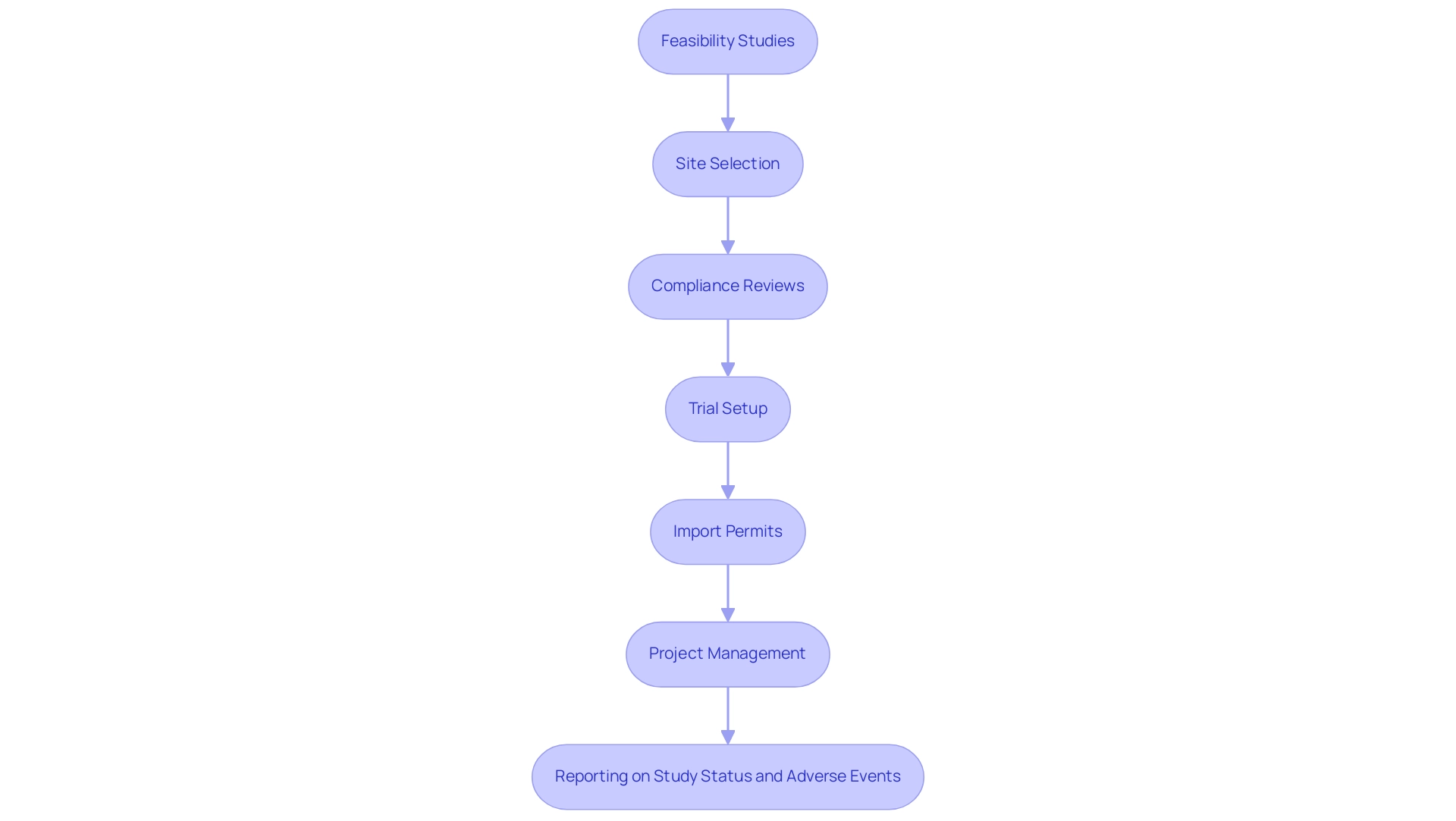
The environment of validation in clinical data management is being reshaped through the use of advanced technologies that greatly improve both precision and productivity. Our comprehensive clinical trial management services include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting on study status and adverse events
Electronic information capture (EDC) systems further facilitate real-time entry and monitoring, effectively minimizing the manual errors often associated with traditional methods.
By integrating automation tools, these systems perform checks and generate alerts for discrepancies, thereby streamlining the validation in clinical data management process. Notably, EDC systems lead to significant cost savings and shorter study timelines, making them a vital asset in research. Furthermore, the incorporation of machine learning algorithms is paving the way for predictive analytics, which proactively identifies potential issues before they escalate.
As Laura Acosta, VP of Product Management in the Clinical Division, succinctly states,
Continuous learning and innovation are essential for driving progress in research.
These innovations not only speed up the validation in clinical data management process but also strengthen integrity, ultimately aiding in the robust execution of trials. For instance, our services enhance patient engagement through user-friendly interfaces for data reporting, fostering a sense of connection and commitment among participants, which leads to higher retention rates and more robust data collection.
Furthermore, adherence to regulatory requirements is essential for research, and our EDC systems include features to monitor changes and document protocols. By embracing these modern technological advancements, organizations can ensure compliance and enhance patient engagement, which are critical for success in research. Ultimately, these services contribute to job creation, economic growth, and healthcare improvement in local communities, emphasizing the significant impact of Medtech research studies.
The Importance of Validation in Clinical Trials: Ensuring Data Quality and Reliability
The process of validation in clinical data management plays a crucial role in clinical trials, fundamentally influencing both information quality and reliability. High-quality information is vital for drawing sound conclusions and making informed decisions regarding patient safety and treatment efficacy. Insufficient validation in clinical data management can lead to significant consequences, such as misinformed treatment protocols and compromised patient safety, ultimately undermining the integrity of the entire study.
Therefore, researchers must rigorously engage in validation in clinical data management to bolster the credibility of their findings, facilitate regulatory approvals, and contribute meaningfully to advancements in medical knowledge. This is particularly pressing in Latin America, where Medtech companies face unique challenges such as regulatory hurdles and the need for effective collaboration with local healthcare institutions. Our comprehensive clinical study coordination services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project oversight
are designed to address these challenges.
For example, our latest case studies illustrate the effect of customized project coordination strategies on enhancing information integrity and experiment efficiency. A culture of ongoing enhancement, emphasized by these examples, underscores the importance of routinely assessing information handling practices and investing in employee training. This proactive approach not only guarantees efficient information management but also improves the overall success of trials.
Furthermore, the National Institutes of Health (NIH) states, 'Collaborative research efforts can accelerate the development of new treatments by 15%,' highlighting the critical role of validation in clinical data management to ensure optimized research reliability and better outcomes for patients. For those interested in further insights, WCG's 2024 Clinical Research Site Challenges Report is now available, providing timely context on current challenges and best practices, particularly focusing on validation in clinical data management in relation to the regulatory environment in Colombia.
Conclusion
In the realm of clinical research, the significance of data validation cannot be overstated. This article has outlined the essential components of clinical data validation, emphasizing the need for accuracy, completeness, and consistency throughout the research lifecycle. By implementing robust validation processes, researchers can ensure the integrity of their data, which is crucial for drawing reliable conclusions and safeguarding patient welfare.
The discussion highlighted best practices, such as:
- Developing comprehensive validation plans
- Utilizing advanced technologies like electronic data capture systems and machine learning algorithms
These innovations not only streamline the validation process but also enhance data quality, ultimately leading to more successful clinical trial outcomes. Additionally, the importance of regulatory compliance has been underscored, stressing that adherence to guidelines established by authorities like the FDA and EMA is vital for maintaining research integrity and participant safety.
As the landscape of clinical trials continues to evolve, the integration of modern techniques and a commitment to continuous improvement remain essential. By embracing these strategies, researchers can navigate the complexities of clinical data validation effectively, ensuring that their findings contribute meaningfully to medical knowledge and the advancement of healthcare solutions. Ultimately, the collective efforts to uphold data integrity will play a pivotal role in enhancing the credibility of research findings and promoting trust in clinical trials.
Frequently Asked Questions
What is the purpose of validation in clinical data management?
The purpose of validation in clinical data management is to ensure the accuracy, completeness, and consistency of information collected during studies, confirming integrity and adherence to regulatory standards.
What are the key concepts involved in validation in clinical data management?
The key concepts include accuracy, completeness, consistency, and timeliness, which are essential for upholding the credibility of research outcomes.
What services are included in trial oversight?
Trial oversight services include feasibility studies, site selection, compliance evaluations, trial setup, project coordination, and reporting.
How is reporting managed in clinical trials?
The reporting process includes detailed communication of study status, inventory management, and documentation of serious and non-serious adverse events to maintain transparency and compliance.
What technology is commonly used in validation processes?
SaS (Statistical Analysis System) is extensively employed for its strong capabilities in analysis, validation, and decision support, which are essential for improving verification processes.
What trends are emerging in healthcare information verification for 2024?
There is a significant emphasis on verification precision, with advancements in methodologies contributing to heightened standards. Additionally, 60% of pharmaceutical firms are investigating blockchain technology to enhance information security.
Why is the role of INVIMA important in clinical research?
INVIMA, as a Level 4 health authority by PAHO/WHO, oversees medical device regulation in Colombia, ensuring compliance with national standards and facilitating the import permit and nationalization of investigational devices.
How does the integration of genomics and personalized medicine relate to clinical data management?
The integration of genomics and personalized medicine exemplifies the transformative potential of precise information management, enabling researchers to analyze genetic markers linked to diseases for more effective and targeted therapies.
What are the critical steps in the validation process?
Critical steps include entry verification, consistency checks, and conducting thorough audits to validate the overall quality of the information.
What best practices can enhance the validation process?
Developing a comprehensive validation plan, incorporating routine checks, adopting automated systems, and providing consistent training for personnel are essential best practices.
What is the significance of Good Data Management Practices (GCDMP)?
Implementing GCDMP requires a commitment that provides substantial benefits for research, making it an essential focus for all stakeholders involved in clinical trials.




