Overview
Latin America is an attractive location for clinical trials due to its cost-effectiveness, diverse patient populations, and robust regulatory frameworks, which enhance the reliability and efficiency of medical studies. The article highlights that strategic partnerships, such as those between bioaccess™ and Caribbean Health Group, not only facilitate streamlined study management but also address local health challenges, thereby improving both research outcomes and community health.
Introduction
Latin America is emerging as a pivotal hub for clinical trials, offering a unique blend of advantages that appeal to researchers and sponsors alike. With initiatives aimed at bolstering the region's reputation for clinical research, such as the collaboration between bioaccess™ and Caribbean Health Group, countries like Colombia are positioning themselves as prime locations for innovative studies.
The region's cost-effectiveness, diverse patient populations, and robust regulatory frameworks not only enhance the potential for successful trial outcomes but also contribute to the local economy. However, navigating the complexities of regulatory requirements, cultural nuances, and ethical considerations remains essential for maximizing the benefits of clinical research in this dynamic landscape.
As the demand for effective health interventions grows, understanding the intricacies of conducting trials in Latin America becomes increasingly critical for stakeholders in the global health arena.
The Advantages of Conducting Clinical Trials in Latin America
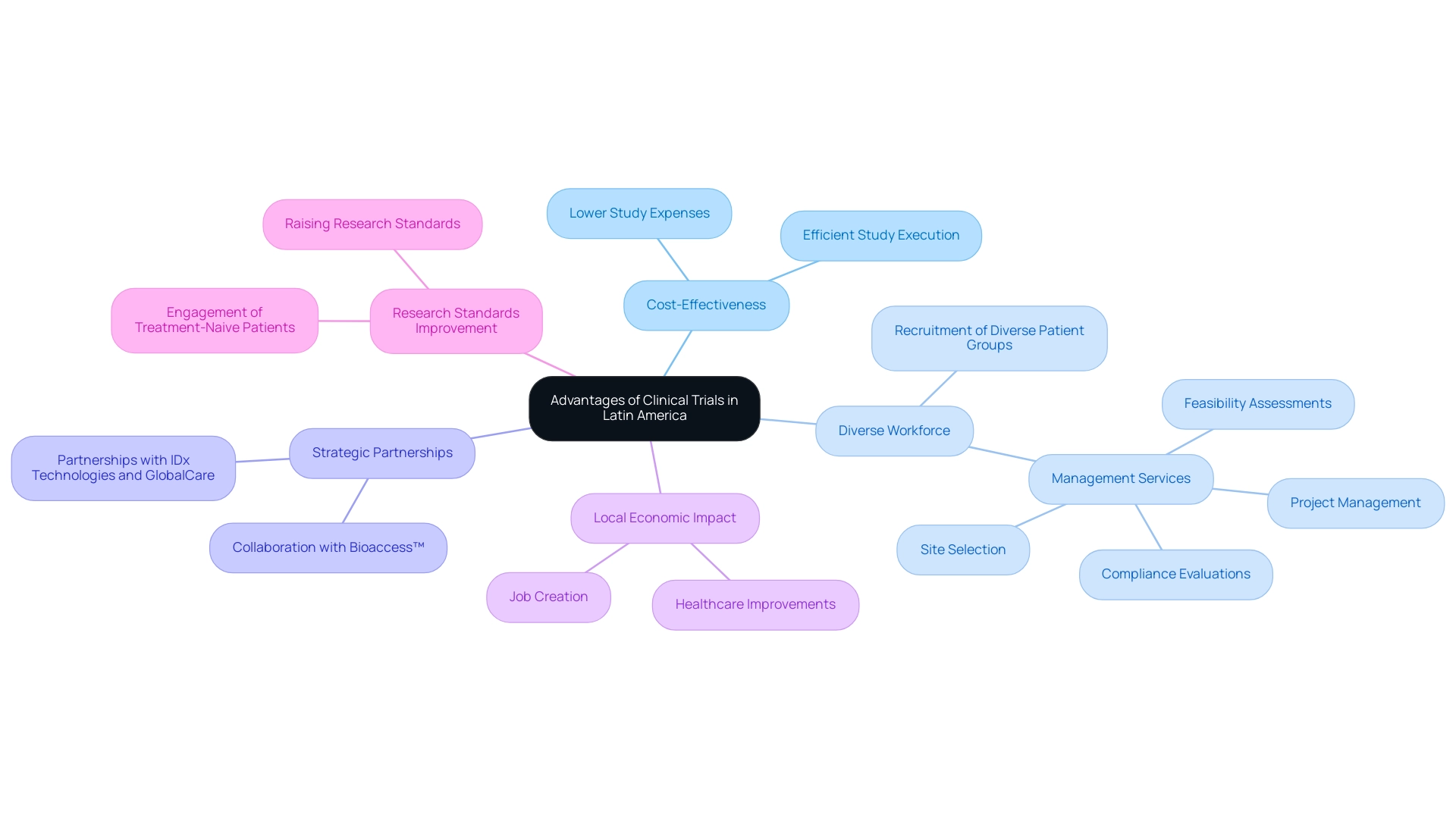
The region offers numerous benefits for carrying out medical studies, which raises the question: why choose Latin America for clinical trials, particularly through strategic partnerships such as that of bioaccess™ and Caribbean Health Group, with the goal of making Barranquilla a top location for health investigations in the area. Backed by Colombia's Minister of Health, this initiative not only improves the reliability of study results by engaging a growing population of treatment-naive patients but also represents a dedication to raising research standards in South America. The region's cost-effectiveness is a key reason why choose Latin America for clinical trials, as it offers considerably lower study expenses compared to North America and Europe, supported by well-established research organizations (CROs) that guarantee efficient study execution.
The proficient workforce and cultural variety in Latin America are key factors in answering the question of why choose Latin America for clinical trials, as they aid in the recruitment of diverse patient groups essential for the applicability of research outcomes. Additionally, bioaccess™ provides extensive management services for studies, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project management
- Reporting
Partnerships with organizations like IDx Technologies and GlobalCare Clinical Trials illustrate the potential for innovation in the Medtech space, with reported achievements such as over a 50% reduction in recruitment time and exceptional retention rates.
These collaborations not only promise timely insights and significant cost savings but also contribute to local economies through job creation and healthcare improvements, reflecting a growing commitment to addressing health challenges in the region.
However, it's vital to acknowledge the broader health challenges that the region faces, such as food insecurity and environmental health issues, as highlighted in the 2023 Report of The Lancet Countdown. This highlights the significance of effective health interventions and the role of corporate involvement in promoting health initiatives, ultimately enhancing the research environment in South America.
Key Factors Driving Clinical Trials in Latin America
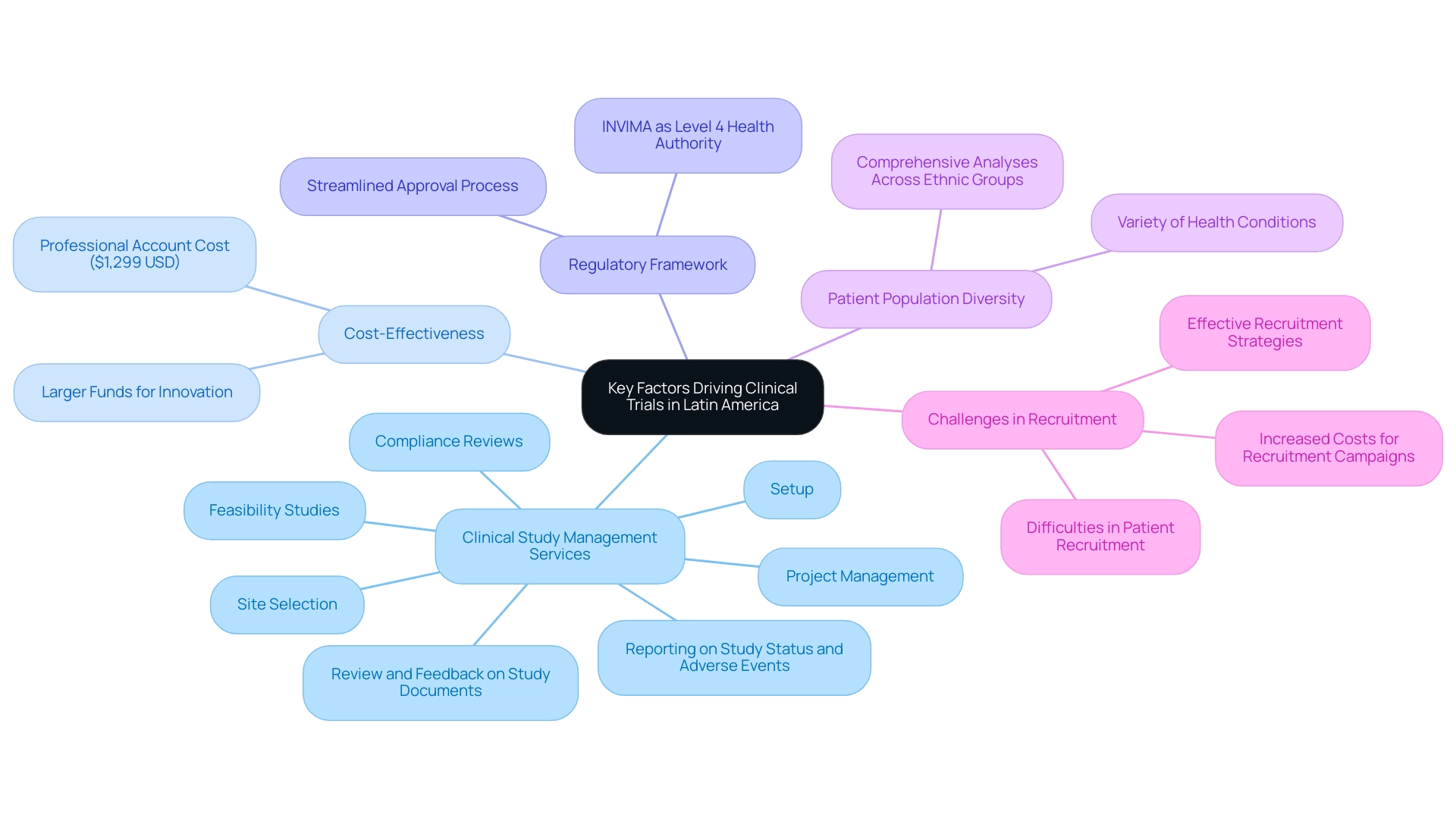
There are several pivotal factors contributing to the growth of clinical studies in Latin America, particularly in Colombia, prompting the question: Why choose Latin America for clinical trials, given its comprehensive clinical study management services? These services include:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Review and feedback on study documents
- Project management
- Reporting on study status and adverse events
These services ensure a streamlined process for sponsors. Importantly, one reason why choose Latin America for clinical trials is that carrying out experiments in this area is considerably more economical than in advanced nations, allowing sponsors to assign larger funds to essential aspects of innovation and development.
For example, the expense of managing a professional account for medical study groups is approximately $1,299 USD, illustrating the cost-effectiveness of activities here. Colombia boasts a robust regulatory framework, leading to the important question of why choose Latin America for clinical trials, as the INVIMA serves as a Level 4 health authority by PAHO/WHO, streamlining the approval process and ensuring compliance without unnecessary delays. This regulatory environment promotes a supportive atmosphere for investigation.
Furthermore, the diversity of patient populations across Latin America enhances the study landscape, which illustrates why choose Latin America for clinical trials, as it allows for comprehensive analyses on treatment effects across various ethnic groups and health conditions. With an ambitious science, technology, and innovation strategy for 2022–2031 aimed at becoming a knowledge economy, Colombia acknowledges these advantages and is strengthening its role in global research, which raises the question of why choose Latin America for clinical trials. Furthermore, although there are difficulties in patient recruitment for research studies, tackling these can greatly enhance study results.
For additional insights into navigating these challenges, Patricio Ledesma, the Head of Operations and Founder at Sofpromed CRO, is a key contact in the field, reachable at +34 607 939 266 or via email at pledesma@sofpromed.com, and can assist sponsors in leveraging Colombia's research advantages.
Challenges and Considerations in Latin American Clinical Trials
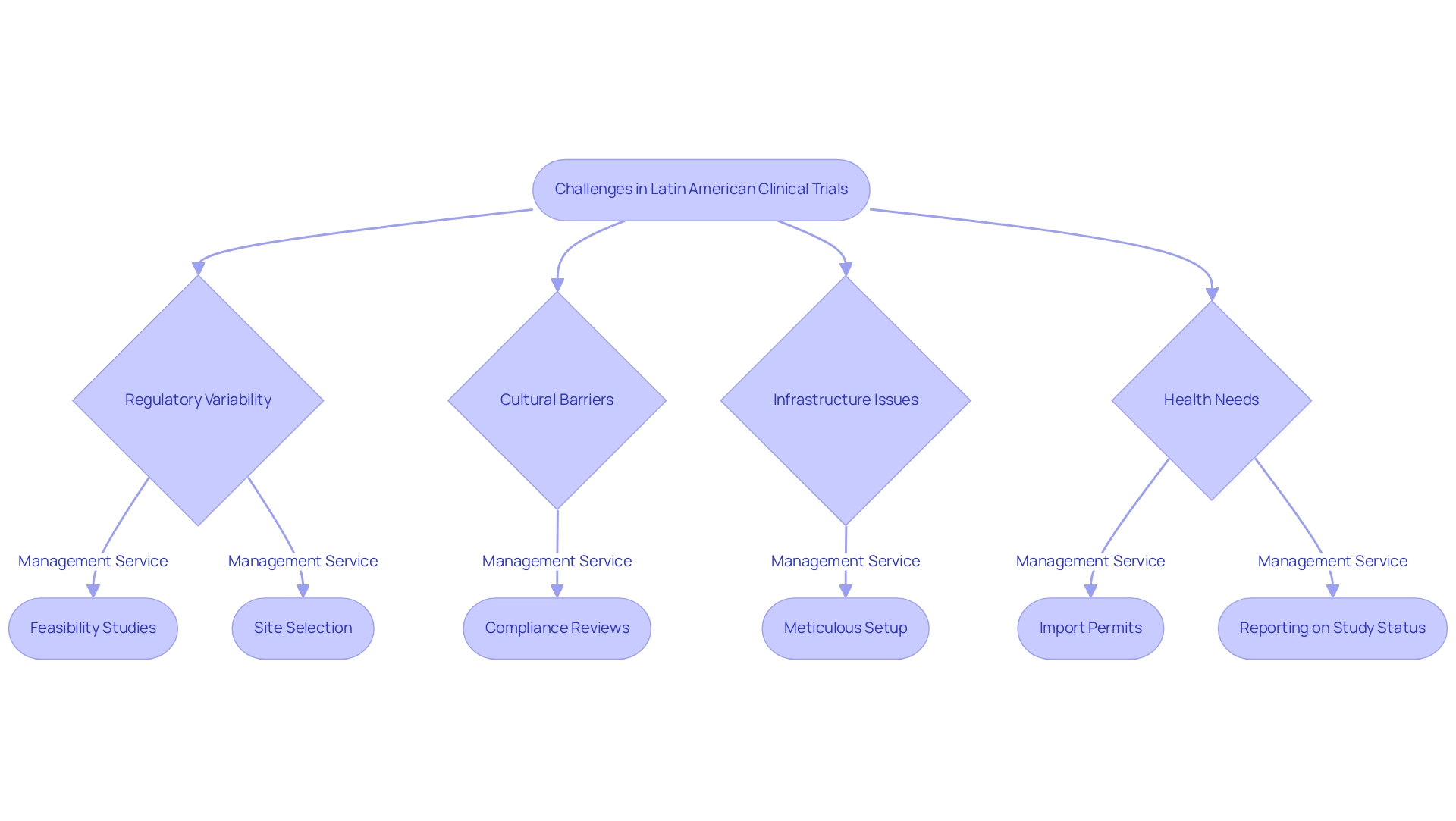
When considering significant opportunities for research studies, one might ask, why choose Latin America for clinical trials, as successfully managing the associated challenges is crucial. A key factor is the variability in regulatory requirements across different countries, which can complicate the approval process. Our comprehensive research management services include:
- Feasibility studies
- Thorough site selection
- Compliance reviews
- Meticulous setup
- Import permits and nationalization of investigational devices
- Reporting on study status and adverse events
This ensures the highest standards of regulatory adherence.
For example, as highlighted by Kendle, a CRO focusing on study management, average approval durations can vary from 14 to 16 weeks, stressing the importance of strict adherence. Furthermore, understanding INVIMA, the Colombia National Food and Drug Surveillance Institute, and its classification as a Level 4 health authority by PAHO/WHO is crucial for compliance and oversight in medical device regulation. Cultural and language barriers also pose challenges, particularly in regions with diverse populations; this is why choosing Latin America for clinical trials is crucial, as exemplified by Mexico City, which has 323,000 indigenous language speakers representing 39 different languages, underscoring the need for culturally sensitive recruitment strategies.
Our research management services are intended to tackle these challenges by utilizing customized recruitment strategies that honor cultural nuances. Understanding why to choose Latin America for clinical trials is important, as the region's varied educational and socioeconomic landscape complicates recruitment efforts, making robust project management and monitoring essential. Infrastructure challenges in certain areas can hinder trial logistics and potentially cause delays.
Furthermore, the increasing occurrence of illnesses such as cancer and diabetes in nations like Chile and Peru emphasizes the urgency for research studies, particularly for vaccine testing in treatment-naive populations, which raises the question of why choose Latin America for clinical trials, given the complexities involved in addressing the specific needs of diverse patient groups. Successfully tackling these challenges, while also guaranteeing data integrity and quality assurance systems, is crucial to fully utilize the potential of medical studies in this dynamic area.
Ethical and Regulatory Considerations for Trials in Latin America
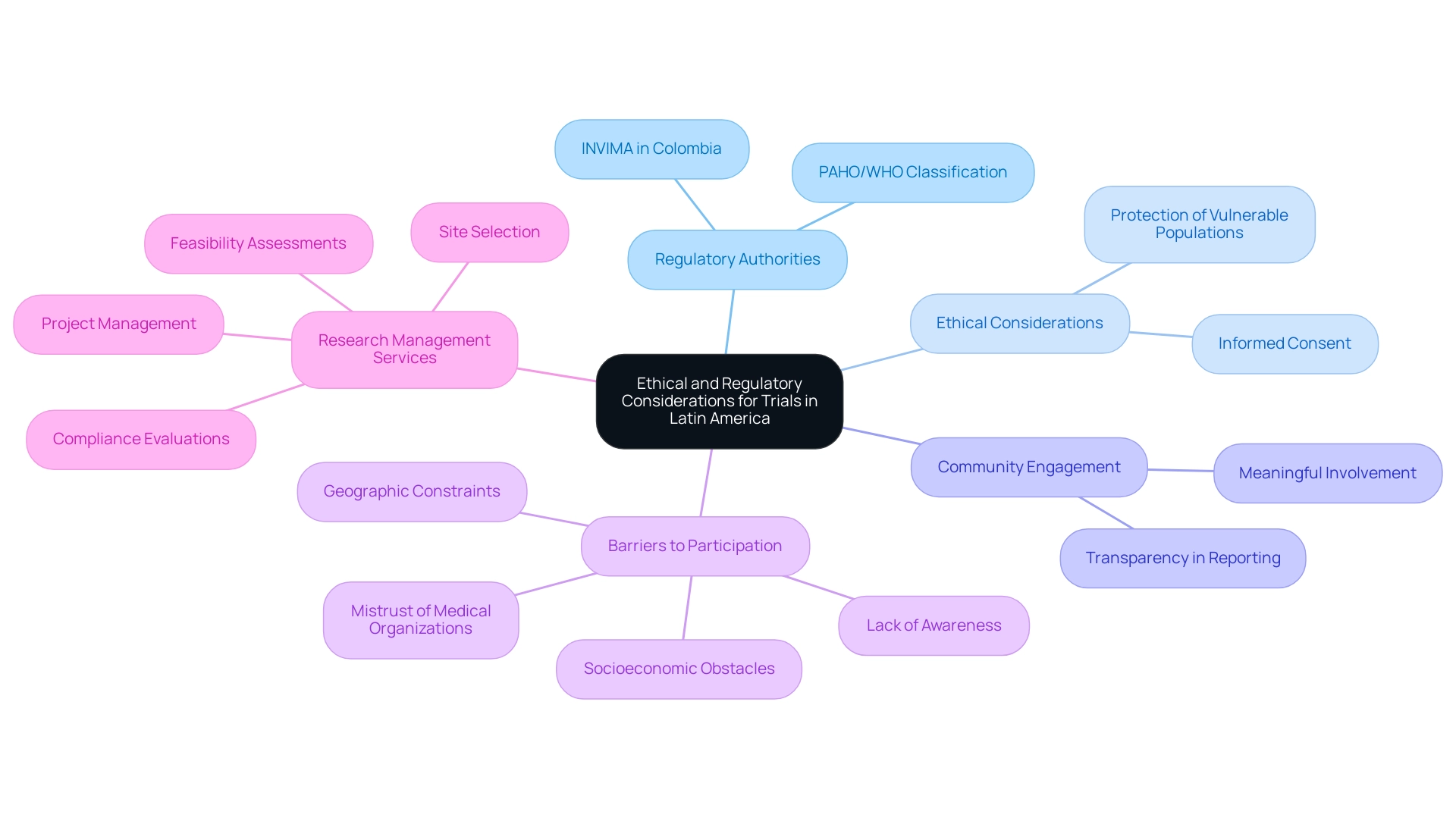
When considering medical studies, one must understand why choose Latin America for clinical trials, as it requires a thorough comprehension of both ethical and regulatory factors that differ from one nation to another. Each country has its own regulatory authority responsible for supervising clinical studies, making adherence to local laws essential for successful execution. In Colombia, for instance, the INVIMA serves as the national authority overseeing medical devices, classified as a Level 4 health authority by PAHO/WHO, which underscores the importance of familiarity with its regulations.
Ethical considerations, especially informed consent and the protection of vulnerable populations, must take precedence to uphold the integrity of the study process. Cooperation with local organizations and ethics committees is essential in guaranteeing that studies are carried out ethically and in accordance with international standards. The updated Declaration of Helsinki highlights the necessity for meaningful involvement with communities and research integrity, especially pertinent in the American context.
Furthermore, promoting transparency in result reporting and adherence to Good Clinical Practice (GCP) guidelines, alongside comprehensive reporting mechanisms, are essential steps in building trust with stakeholders and the public. Recognizing the challenges—such as the fact that only 4% of cancer studies are currently ongoing in Latin America—brings to light why choose Latin America for clinical trials, highlighting the need for a concerted effort to enhance awareness and participation. Significantly, surveys show that over 80% of individuals would contemplate joining a study if they were informed of the opportunities, emphasizing the importance of tackling barriers to participation.
Moreover, elements like lack of awareness, deep mistrust of medical organizations, socioeconomic obstacles, and geographic constraints add to the underrepresentation of minority communities in cancer research studies, further complicating the landscape of medical investigation in the area. By leveraging extensive research study management services—including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting—researchers can enhance the effectiveness and reach of their studies, ultimately benefiting local economies through job creation, healthcare improvement, and international collaboration. Katherine Ruiz, a specialist in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, highlights the significance of navigating these regulatory landscapes to ensure successful results.
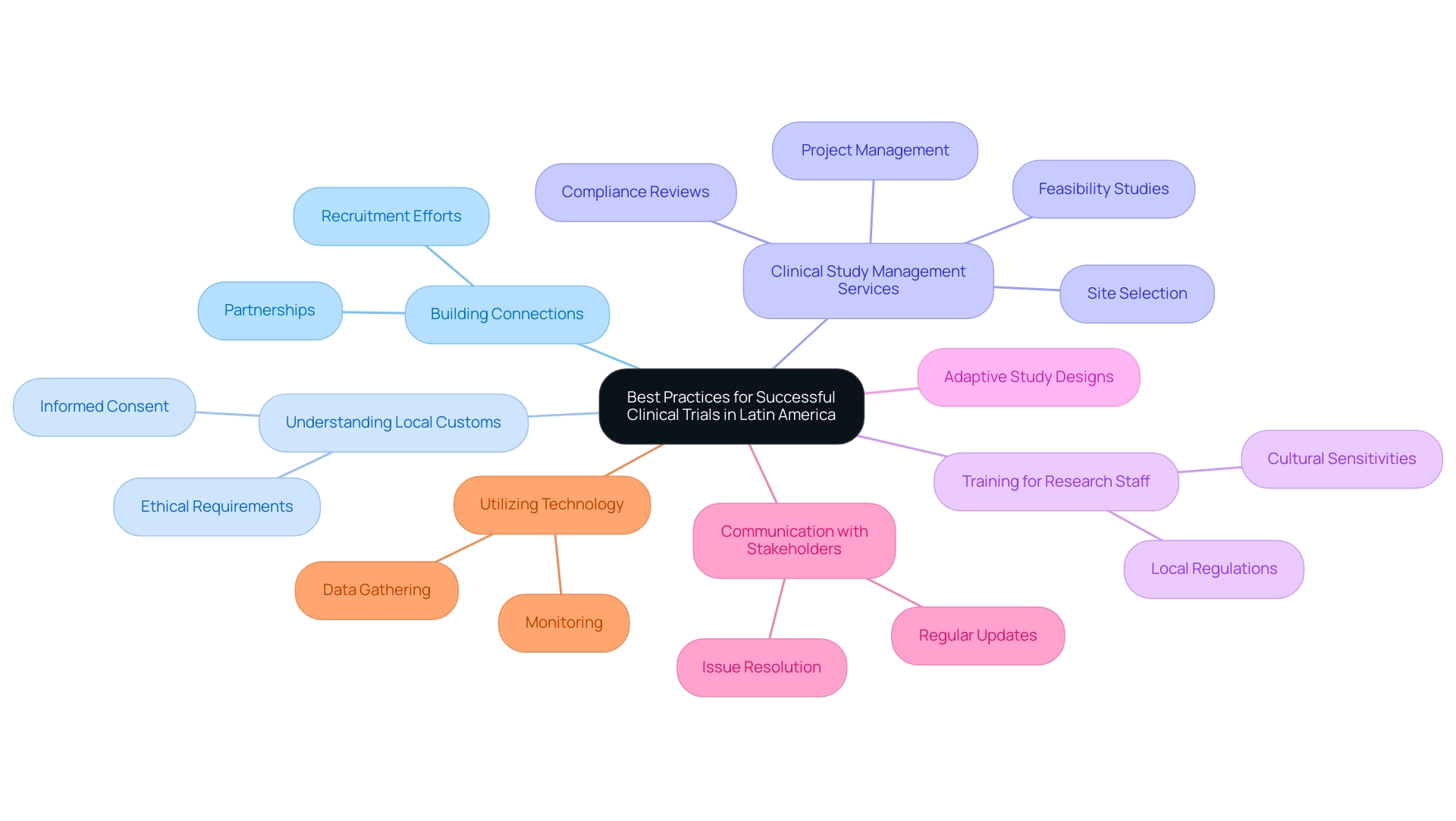
Best Practices for Successful Clinical Trials in Latin America
To attain successful medical studies in Latin America, one must understand why choose Latin America for clinical trials and follow several best practices. Building strong connections with local researchers and organizations, as shown by the partnership between bioaccess™ and Caribbean Health Group to establish Barranquilla as a premier location for clinical studies, greatly improves recruitment efforts and simplifies study management. Dr. Colin Scott, a senior pharmaceutical industry executive, emphasizes this importance, stating, 'For many studies, this could be the difference between a swift FDA approval or prolonged delays.'
Moreover, understanding local customs and ethical requirements for informed consent is vital, as emphasized in the case study titled 'Respect for Local Culture and Ethics,' which demonstrates that sponsors who honor local practices and work with regional experts are more likely to achieve successful outcomes. The recent backing from Colombia's Minister of Health for expanding research projects highlights this collaborative effort. Comprehensive clinical study management services, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, are vital for ensuring the efficiency and success of these studies.
Additionally, the collaboration has led to over a 50% reduction in recruitment time and a remarkable 95% retention rate, showcasing its effectiveness. Investing in thorough training for research staff on local regulations and cultural sensitivities fosters smoother operational processes. Considering that merely 9% of the population in Boise, Idaho, is Latin American, there is considerable potential for diverse participant recruitment in studies carried out in this area.
The implementation of adaptive study designs can further promote flexibility and responsiveness to challenges encountered during the studies. Regular communication with all stakeholders, including sponsors and regulatory bodies, is vital for maintaining alignment and addressing issues promptly. Ultimately, utilizing technology for data gathering and monitoring not only improves efficiency but also guarantees data integrity throughout the process, which is essential for successful outcomes.
Notably, one might wonder why choose Latin America for clinical trials, as it is well-suited for conducting infectious disease studies due to seasonal variations in disease occurrence, making it an advantageous region for such trials.
Conclusion
Latin America is rapidly establishing itself as a critical player in the global clinical trials landscape, driven by a unique combination of cost-effectiveness, diverse patient populations, and robust regulatory frameworks. The collaboration between organizations such as bioaccess™ and Caribbean Health Group exemplifies the region's commitment to enhancing clinical research standards while fostering local economic growth. As highlighted, Colombia's strategic initiatives and supportive regulatory environment provide an attractive backdrop for sponsors seeking efficient and effective trial execution.
However, navigating the complexities of conducting clinical trials in this region requires careful attention to regulatory requirements, cultural sensitivities, and ethical considerations. Understanding the nuances of local regulations and fostering relationships with local investigators can significantly enhance recruitment efforts and streamline trial management processes. By addressing the challenges related to patient recruitment and ensuring compliance with ethical standards, stakeholders can maximize the benefits of clinical research while contributing to the advancement of health interventions in Latin America.
As the demand for innovative health solutions continues to rise, the potential for successful clinical trials in Latin America is immense. Stakeholders must leverage the region's advantages while remaining vigilant about the challenges that accompany such opportunities. Emphasizing collaboration, cultural respect, and comprehensive management practices will be key to realizing the full potential of clinical research in this dynamic and diverse region. The commitment to improving health outcomes through rigorous and ethical clinical trials not only promises advancements in medical science but also enriches the communities involved, paving the way for a healthier future.




