Overview
Colombia is emerging as the premier destination for medtech trials, driven by its streamlined regulatory processes, diverse patient recruitment opportunities, and competitive cost structure. These factors collectively enhance the efficiency and effectiveness of clinical studies.
The article highlights that:
- Colombia's favorable regulatory environment
- Robust healthcare infrastructure
- Attractive R&D incentives
These elements facilitate quicker approvals and lower operational costs. Moreover, they support innovation, making Colombia an appealing choice for U.S. medical device firms aiming to conduct research.
Introduction
Colombia is rapidly emerging as a leading hub for Medtech clinical trials, driven by a confluence of strategic advantages that attract companies eager to innovate in the medical technology sector. The country features streamlined regulatory approval processes, resulting in some of the fastest timelines for medical device and pharmaceutical approvals in Latin America. This efficiency enables companies to accelerate their time-to-market. Additionally, Colombia's diverse population presents abundant patient recruitment opportunities, complemented by a robust hospital infrastructure that provides fertile ground for clinical research.
Financial incentives established by the Colombian government, including significant tax credits for research and development, further enhance the appeal for Medtech firms looking to invest in this dynamic landscape. As global demand for clinical trials evolves, Colombia stands at the forefront, poised to support the next wave of medical advancements while contributing to local economies and improving healthcare outcomes.
Streamlined Regulatory Approval Processes
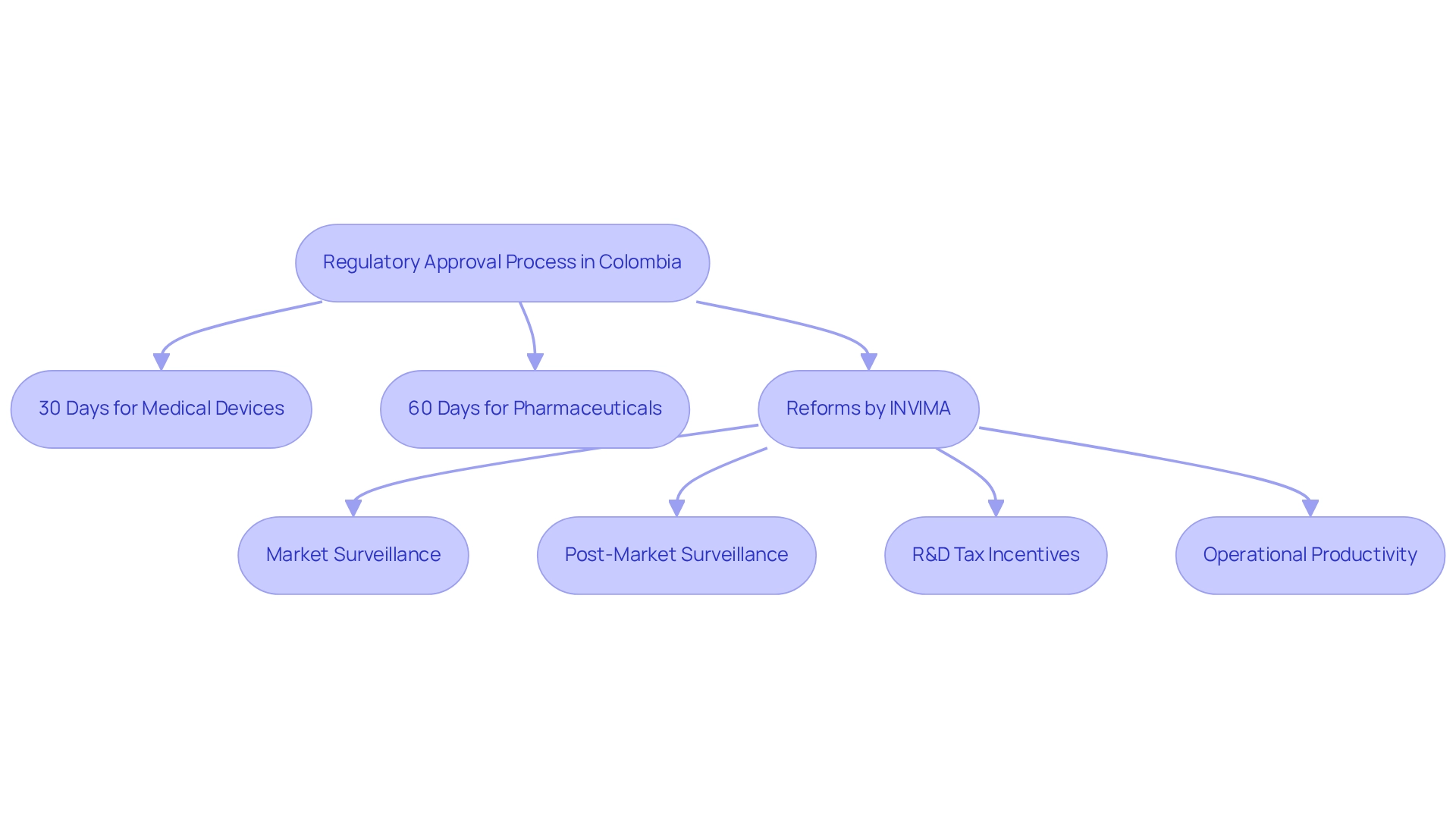
Colombia is recognized for having one of the most effective regulatory approval processes in Latin America, featuring a national timeline of just 30 days for medical devices and 60 days for pharmaceuticals. This impressive speed is largely due to the reforms instituted by INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), which has significantly streamlined the review process. As a Level 4 health authority classified by PAHO/WHO, INVIMA empowers companies to drastically reduce their time-to-market, allowing them to capitalize on new opportunities more swiftly than in regions encumbered by more complex regulatory frameworks.
This efficiency not only boosts operational productivity but also fosters a more favorable environment for innovation within the Medtech sector. Julio G. Martinez-Clark, CEO of bioaccess™, states, "The blend of a substantial and varied population, skilled research facilities, effective regulatory procedures, a cost-effective setting, and a history of successful medtech studies since 2010 contribute to making Colombia as a medtech trial destination an appealing option for U.S. medical device firms." bioaccess™ offers a range of services including regulatory consulting, market access strategies, and study management, positioning itself as a vital ally for navigating the Colombian healthcare landscape.
Moreover, the Colombian healthcare equipment market, valued at $1.42 billion in 2022, highlights the potential for growth and investment in this vibrant sector. The incorporation of robust market surveillance and post-market surveillance practices further fortifies the regulatory framework, ensuring that medical devices are not only approved swiftly but also monitored effectively after approval. Additionally, U.S. medical device firms can benefit from R&D tax incentives, which provide substantial financial advantages, thereby enhancing Colombia's appeal as a medtech trial destination and making it an even more attractive location for research studies.
Abundant Patient Recruitment Opportunities
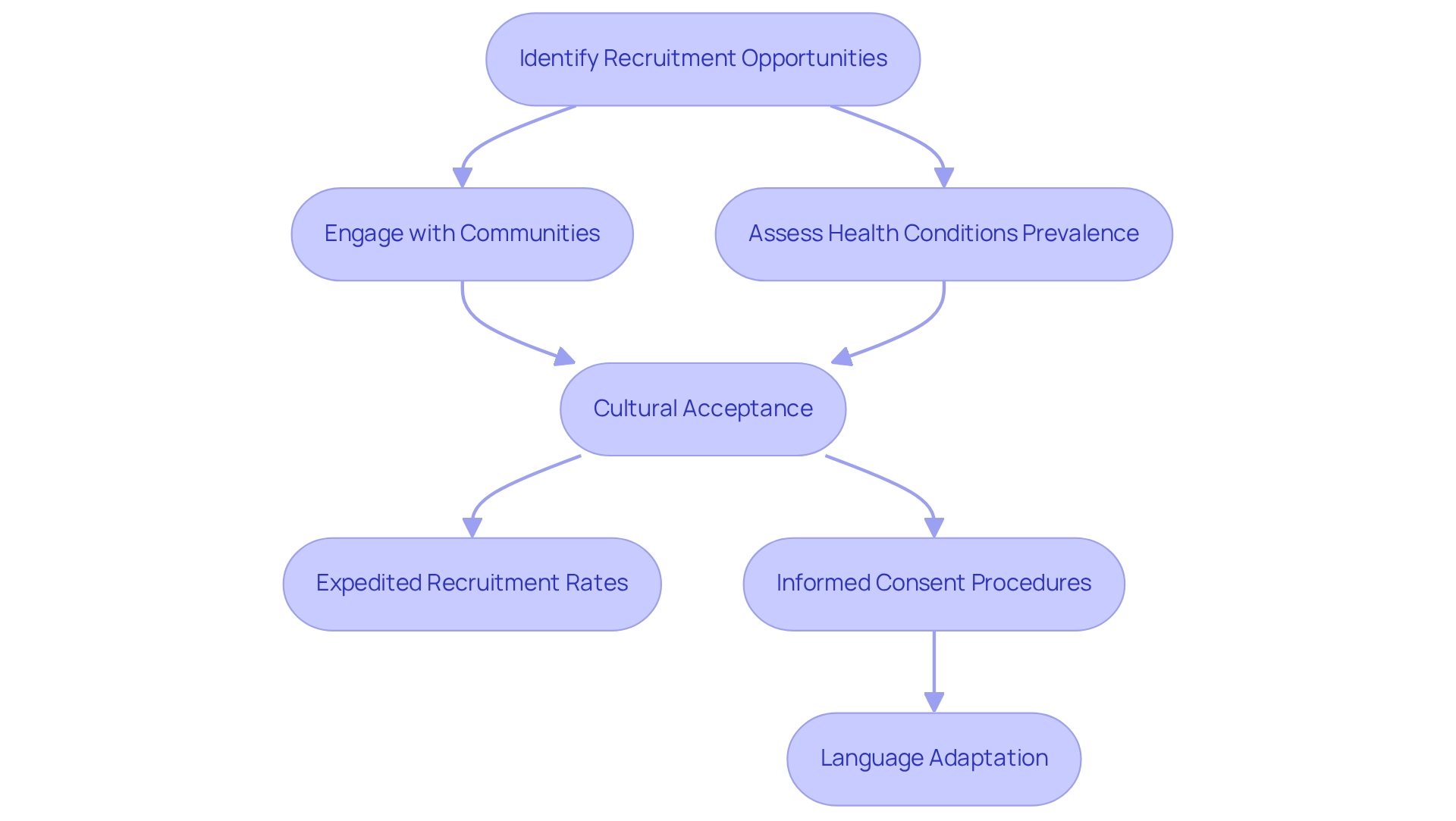
With a diverse population exceeding 50 million, Colombia stands out as a prime medtech trial destination, presenting substantial opportunities for patient enrollment. The country exhibits a significant prevalence of various health conditions, facilitating the selection of suitable participants for clinical studies. Notably, the cultural acceptance of medical research plays a crucial role in enhancing patient willingness to participate, which is essential for achieving expedited recruitment rates.
This aspect proves particularly beneficial for early-phase studies, where timely enrollment is vital for success.
Furthermore, leveraging local networks and engaging with communities can significantly enhance recruitment strategies. By fostering connections within these communities, Medtech firms can refine their recruitment approaches and ensure that their studies accurately reflect the diverse patient demographics in the country. This strategy not only aids in effectively meeting study objectives but also aligns with the ethical considerations of conducting research in varied cultural contexts.
As Julio G. Martinez-Clark, CEO of bioaccess®, observes, 'The government of Colombia as a medtech trial destination appears to be the sole nation in Latin America with a proactive strategy to draw more research studies as part of its goal to transform into a knowledge economy by 2031.' This initiative underscores the supportive environment for medical research within the nation.
Nonetheless, challenges persist, particularly concerning informed consent procedures. While literacy rates in neighboring Chile are reported at 95.7%, it is crucial to acknowledge that differing literacy levels across the region can affect patient comprehension and consent. Additionally, language barriers pose significant challenges for studies originating from outside Latin America.
Regulatory documents must be provided in Castilian Spanish, while patient materials require adaptation to local dialects and cultural nuances. Effectively addressing these linguistic and cultural differences is paramount for ethical treatment and informed consent, helping to prevent misunderstandings and delays in the approval process.
To adeptly navigate these challenges, bioaccess® offers comprehensive trial management services, including Early-Feasibility Studies, First-In-Human Studies, and Pilot Studies, ensuring that trials are executed efficiently and ethically while adhering to local regulatory standards.
Robust Hospital Infrastructure Supporting Trials
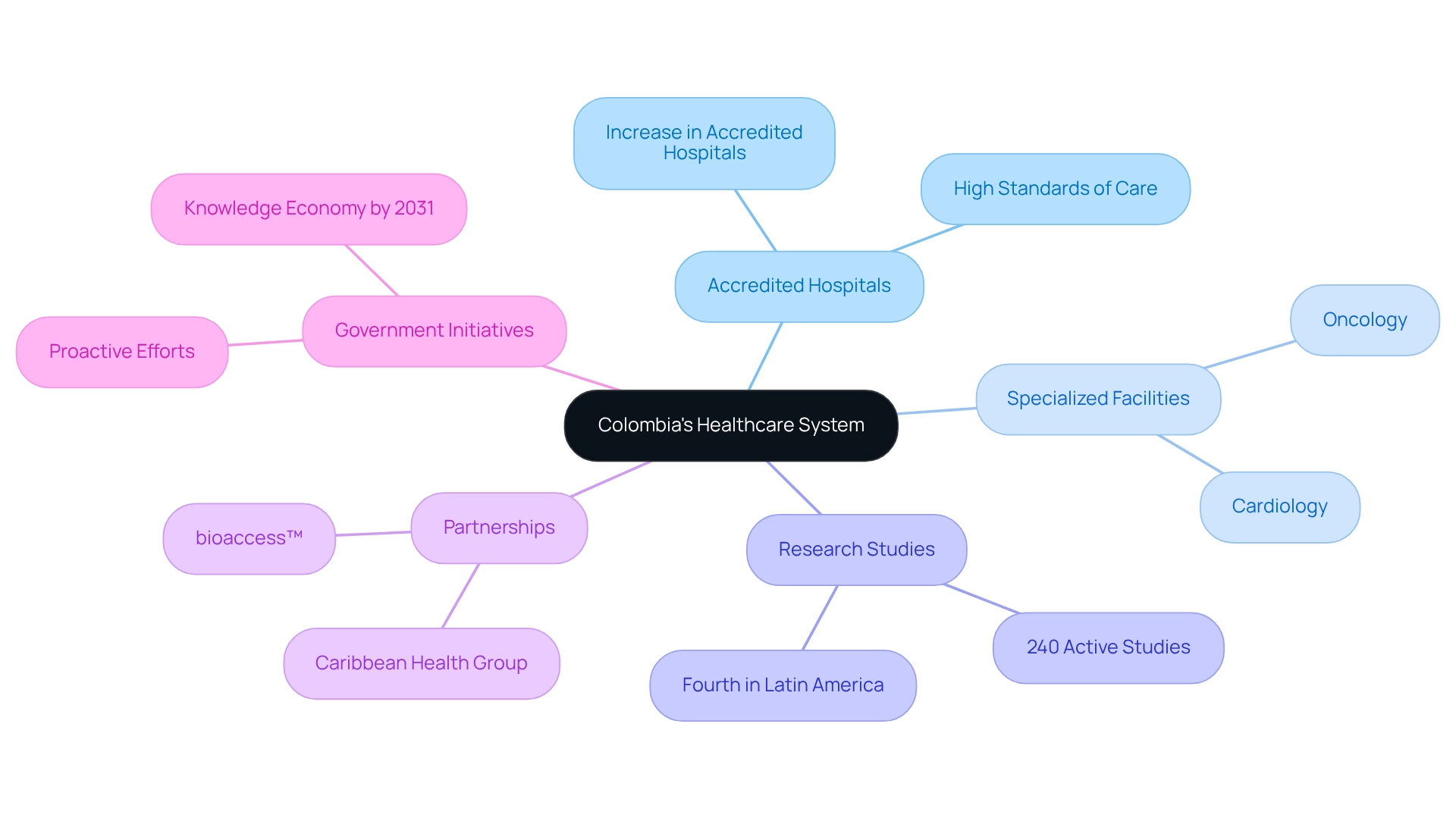
Colombia boasts a sophisticated healthcare system, characterized by a network of accredited hospitals equipped with cutting-edge medical technologies and facilities. These institutions are recognized for their commitment to high standards of care and play a crucial role in research. Specialized facilities in areas such as cardiology and oncology significantly enhance the capacity to conduct intricate medical studies.
This well-established infrastructure is essential for ensuring patient safety and the integrity of medical data, both of which are vital for regulatory submissions and market approvals.
As of 2025, the country has seen a significant increase in the number of accredited hospitals capable of performing research studies, with a focus on enhancing their research capabilities. Currently, approximately 240 research studies are either actively seeking participants or are in the preparatory stages, positioning Colombia as the fourth leading nation in Latin America for medical studies per million individuals. This growth underscores the region's potential, particularly in the Medtech sector, where the demand for reliable and high-quality medical data is critical.
The partnership between bioaccess™ and Caribbean Health Group (CHG), announced on March 29, 2019, aims to establish Barranquilla as a prominent hub for research studies in Latin America, with support from the country's Minister of Health, Juan Pablo Uribe. This initiative is part of a broader strategy to attract more medical study projects to the region. Expert opinions highlight that the country's administration is actively fostering an environment conducive to medical research, with the goal of attracting more studies as part of its larger initiative to evolve into a knowledge economy by 2031.
Julio G. Martinez-Clark, CEO, remarked, "The administration of the country seems to be the sole nation in Latin America with a proactive effort to draw more research studies." This commitment, combined with the existing infrastructure, positions Colombia as a medtech trial destination for companies looking to conduct research studies efficiently and effectively. Moreover, the cost-effective environment in the country facilitates larger studies, benefiting patients and advancing medical knowledge.
GlobalCare Clinical Trials' collaboration with bioaccess™ has already achieved over a 50% reduction in recruitment time and 95% retention rates, demonstrating the effectiveness of these collaborative efforts.
Competitive Cost Structure for Clinical Research
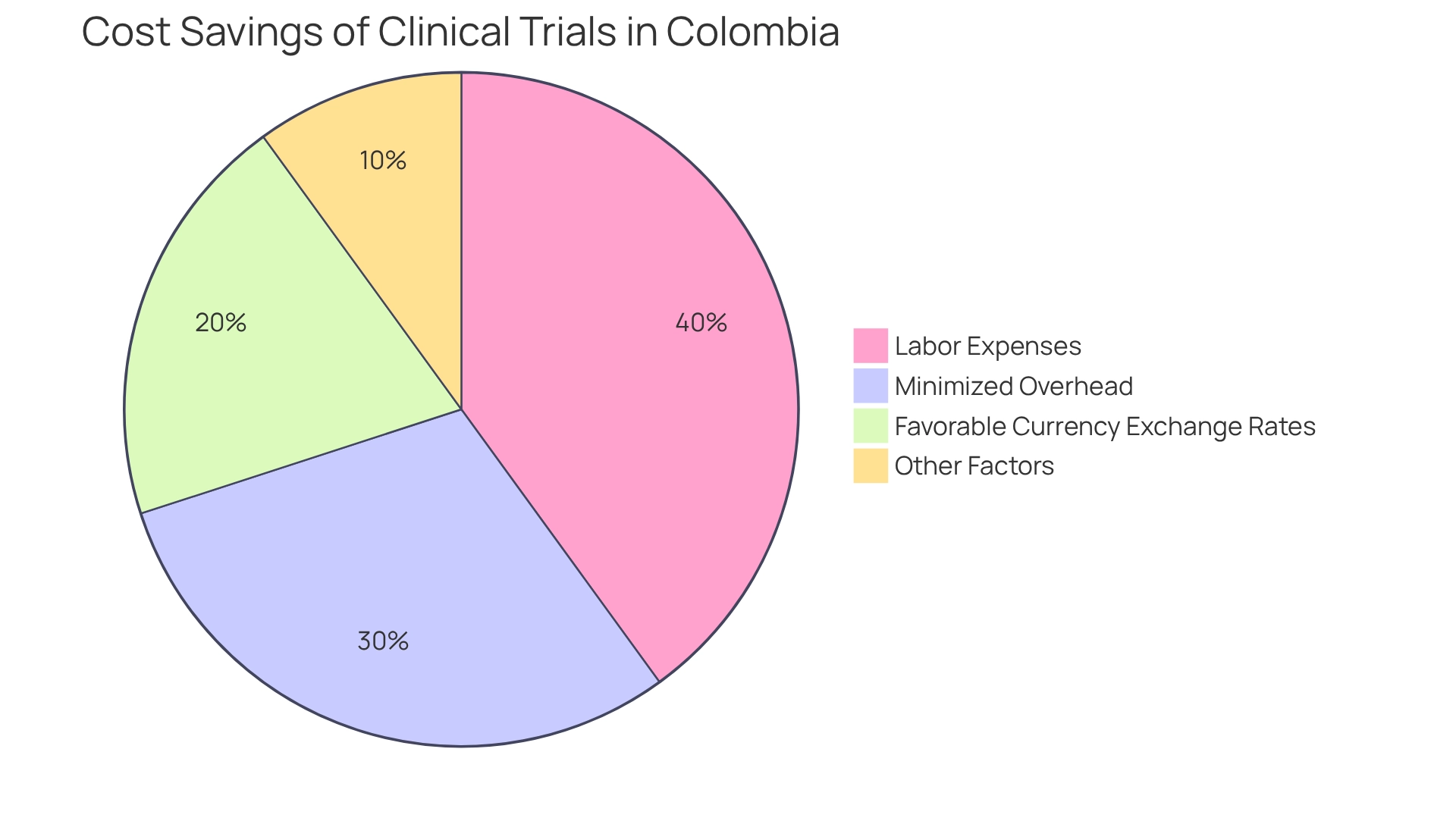
Carrying out medical trials in Colombia as a medtech trial destination presents a significant opportunity for Medtech firms, offering cost reductions that can range from 30% to 75% compared to North America and Europe. This remarkable decrease in operational costs stems from several key factors, including lower labor expenses, minimized overhead, and favorable currency exchange rates. Such financial advantages empower Medtech startups to enhance their budgets, allowing for reinvestment into further studies or the expansion of development capabilities.
Moreover, these cost savings do not compromise quality. Colombia is recognized for its commitment to high medical practice standards, ensuring that the integrity of studies is upheld. As highlighted in the case study titled 'Cost-Effective Clinical Studies in Emerging Markets,' conducting studies in Colombia not only offers reduced operational expenses but also provides access to diverse patient populations, which is essential for comprehensive research results.
Bioaccess provides extensive management services for studies, encompassing feasibility assessments, site selection, compliance evaluations, setup, import permits, nationalization of investigational devices, project oversight, and reporting. This robust support network is crucial for Medtech firms striving to navigate the complexities of research processes in South America. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, shares his positive experience with bioaccess during its initial human testing in South America, emphasizing the efficiency of their services in facilitating successful research outcomes.
Patricio Ledesma, Head of Clinical Operations and Founder at Sofpromed CRO, underscores the significance of this landscape, expressing his personal and enthusiastic dedication to assisting biotech Chief Executive, Operations, Scientific, Medical, and Regulatory Officers in the planning and execution of phase I-IV clinical studies across North America, Europe, Asia-Pacific, Latin America, and the Middle East. This commitment highlights the operational cost savings for Medtech firms in Colombia as a medtech trial destination.
Additionally, the cost of professional accounts for teams of up to five people is $1,299 USD, illustrating the tangible financial benefits available in this region. Consequently, Medtech companies can conduct their trials efficiently while benefiting from a robust regulatory environment that supports innovation, including the oversight provided by INVIMA, the National Food and Drug Surveillance Institute of the country. This combination of affordability and quality positions Colombia as a premier medtech trial destination for conducting medical research in the Medtech sector.
Accessibility and Ease of Travel for Researchers
Colombia's advantageous geographic position, combined with its robust transportation infrastructure, significantly simplifies travel for international researchers. Major urban centers such as Bogotá, Medellín, and Cali feature international airports that provide direct flights to numerous global destinations, facilitating seamless entry into the country. Furthermore, an extensive internal transport network, encompassing domestic flights and well-maintained road systems, guarantees quick access to research sites throughout the nation.
This streamlined travel experience not only enhances the efficiency of study management but also fosters improved collaboration among teams, sponsors, and local healthcare providers.
At bioaccess, we deliver comprehensive study management services, which include:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
These capabilities are essential for navigating the complexities of conducting clinical research in Latin America, ensuring studies are executed with regulatory excellence and innovation. Our commitment to regulatory excellence guarantees that all studies adhere to local regulations, while our innovative approaches facilitate efficient trial execution.
As highlighted by Vishakha Agrawal from Expert Market Research, 'At Expert Market Research, we aim to bring you the latest insights and trends in the market,' underscoring the importance of understanding the evolving transportation landscape.
The expansion of the public transportation sector in Colombia, driven by changing consumer preferences for sustainability and efficiency, further reinforces this narrative. With government investments in infrastructure and a shift in consumer behavior, the transportation sector is evolving, which is crucial for advancing medical research initiatives.
In a global context, the United States is projected to generate the most revenue in public transportation, amounting to US$52bn in 2025, highlighting the significance of robust transportation systems. Key contacts for infrastructure in Colombia, such as the National Infrastructure Agency and the Colombian Chamber of Infrastructure, provide valuable resources for further exploration, thereby enhancing Colombia as a medtech trial destination as the demand for such investigations in Latin America continues to grow.
The impact of Medtech research studies on local economies, including job creation, economic growth, and healthcare enhancement, underscores the importance of international cooperation in this field.
Attractive R&D Incentives for Medtech Companies
The Colombian government has established a robust framework of R&D incentives aimed at stimulating innovation and attracting foreign investment within the Medtech sector. Notably, small and medium-sized enterprises (SMEs) can access tax credits of up to 50% for qualifying R&D activities, significantly alleviating financial pressures and enhancing their capacity for investment. The design features of these R&D tax incentives are crucial to their effectiveness and accessibility, particularly for SMEs and startups, shaping the overall landscape of business R&D investment.
Moreover, organizations involved in research studies benefit from decreased tariffs on imported medical devices and essential equipment, further reducing operational expenses. As Julio G. Martinez-Clark, CEO of bioaccess Colombia, articulates, 'Colombia as a medtech trial destination is appealing for U.S. medical device companies, thanks to its large and diverse population, experienced medical study sites, efficient regulatory processes, a cost-competitive environment, and a track record of successful medtech evaluations since 2010.' This statement underscores the strategic advantages Colombia offers in the Medtech arena.
These incentives not only diminish the financial burden on Medtech firms but also cultivate a culture of investment in research and development. For instance, bioaccess provides extensive management services for research studies, encompassing feasibility assessments, site selection, compliance evaluations, study preparation, import permits, project oversight, reporting on study progress, and input on study documents. These services are vital for navigating the research landscape in the region. Furthermore, the regulatory responsibilities of INVIMA, as a Level 4 health authority, play a pivotal role in ensuring compliance and facilitating the approval process for research studies.
Consequently, companies are empowered to accelerate advancements in medical technology, enhancing their competitive edge in the global market. The strategic backing from the South American nation is crucial; it not only enables prompt financial assistance but also fosters a sustainable atmosphere for innovation, ultimately establishing Colombia as a premier medtech trial destination.
Strategic Advantages of Choosing Colombia for Medtech Trials
Colombia has emerged as a leading location for Medtech studies, establishing itself as a prominent destination for medical technology trials. This South American nation offers various strategic benefits that make it an appealing choice for firms in the field. Notably, Colombia features efficient regulatory procedures that enable swifter approvals, allowing for quicker commencement of research studies. Additionally, the country provides plentiful patient recruitment prospects, boasting a diverse population that enhances the representativeness of research data.
GlobalCare Clinical Studies achieved more than a 50% reduction in recruitment time and impressive 95% retention rates through collaboration with bioaccess™, underscoring the effectiveness of conducting studies in this region.
The robust hospital infrastructure in Colombia supports high-quality clinical research, equipped with modern facilities and experienced healthcare professionals. This infrastructure, combined with a competitive cost framework, allows Medtech firms to conduct studies more effectively and cost-efficiently compared to conventional markets. The ongoing recruitment crisis in the U.S. further emphasizes the advantages of conducting trials in Colombia, where simpler recruitment and lower expenses present considerable benefits.
Convenient travel logistics within the country further enhance its attractiveness, facilitating smooth accessibility for global sponsors and researchers. Moreover, the Colombian government offers appealing R&D incentives, including a 100% tax deduction for investments in science and technology, a 25% tax reduction, and significant grants, making it financially advantageous for businesses to conduct studies in the area.
As the global clinical studies landscape evolves, Colombia's strategic benefits position it as a crucial player, with 52% of global clinical studies now taking place outside the U.S. Low- and middle-income nations are increasingly recognized for their larger subject pools and fewer regulatory hurdles. This trend underscores the significance of Colombia as a Medtech trial destination, where innovative medical technologies can progress more swiftly and efficiently.
Allison Kalloo, founding partner and communications lead of Clinical Ambassador and iParticipate, notes that the current environment is shaped by a need for enhanced healthcare access and a commitment to diversity in participant enrollment. Furthermore, the impact of medical research contributes to job creation, economic development, and healthcare improvement in local economies, highlighting the importance of conducting trials in the region. The collaboration between bioaccess™ and Caribbean Health Group aims to establish Barranquilla as the premier location for medical research in Latin America, supported by Colombia's Minister of Health, thereby promoting Colombia as a Medtech trial destination and reinforcing the region's role in advancing Medtech innovations.
bioaccess™ specializes in a range of clinical studies, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, all of which are crucial for the advancement of medical technologies.
Conclusion
Colombia is rapidly emerging as a premier destination for Medtech clinical trials, propelled by a range of compelling advantages. The country’s efficient regulatory approval processes facilitate swift timelines in securing approvals for medical devices and pharmaceuticals, thereby enhancing operational efficiency. Moreover, Colombia's diverse population presents rich patient recruitment opportunities, which are crucial for timely enrollment in clinical studies.
The well-established hospital infrastructure supports high-quality research, while a competitive cost structure allows Medtech companies to conduct trials more economically than in traditional markets. This is particularly beneficial in light of recruitment challenges faced in regions such as the U.S., where Colombia offers a more accessible alternative. Additionally, the ease of travel within the country fosters improved collaboration among research teams and local healthcare providers.
Support from the Colombian government through attractive R&D incentives, including significant tax credits and reduced tariffs for research, further enhances the appeal for investment in Medtech. This supportive environment nurtures innovation and provides financial relief for companies.
As the global clinical trials landscape evolves, Colombia's strategic advantages position it as a pivotal player in advancing medical technologies. Collaborations between local organizations, such as bioaccess™ and Caribbean Health Group, further solidify the country's potential to lead in clinical research across Latin America. By selecting Colombia for clinical trials, Medtech companies not only benefit from an efficient research environment but also contribute to improved healthcare outcomes and economic growth in the region.
Frequently Asked Questions
What is the regulatory approval timeline for medical devices and pharmaceuticals in Colombia?
Colombia has a national timeline of just 30 days for medical devices and 60 days for pharmaceuticals.
What organization is responsible for the regulatory approval process in Colombia?
The Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA) is responsible for the regulatory approval process in Colombia.
How has INVIMA improved the regulatory process for medical products?
INVIMA has streamlined the review process, significantly reducing the time-to-market for companies, allowing them to seize new opportunities more quickly.
What advantages does Colombia offer for U.S. medical device firms?
Colombia offers a substantial and varied population, skilled research facilities, efficient regulatory procedures, a cost-effective environment, and a history of successful medtech studies since 2010.
What is the value of the Colombian healthcare equipment market?
The Colombian healthcare equipment market was valued at $1.42 billion in 2022.
What practices strengthen the regulatory framework for medical devices in Colombia?
The incorporation of robust market surveillance and post-market surveillance practices fortifies the regulatory framework, ensuring effective monitoring of medical devices after approval.
What financial incentives are available for U.S. medical device firms in Colombia?
U.S. medical device firms can benefit from R&D tax incentives that provide substantial financial advantages.
Why is Colombia considered a prime medtech trial destination?
Colombia has a diverse population exceeding 50 million, significant prevalence of various health conditions, and a cultural acceptance of medical research, which enhances patient willingness to participate in clinical studies.
What challenges exist regarding informed consent in clinical studies in Colombia?
Challenges include varying literacy levels across the region and language barriers, which can affect patient comprehension and consent.
How can Medtech firms improve recruitment strategies in Colombia?
By leveraging local networks and engaging with communities, Medtech firms can refine their recruitment approaches to better reflect the diverse patient demographics and meet study objectives.
What services does bioaccess® provide to assist with clinical trials in Colombia?
bioaccess® offers regulatory consulting, market access strategies, study management, Early-Feasibility Studies, First-In-Human Studies, and Pilot Studies to ensure trials are executed efficiently and ethically.




