Overview
The article "Exploring Latin America Medical Trial Regions: An Essential Guide for Researchers" emphasizes the increasing importance of Latin America as a pivotal hub for clinical trials. It highlights the region's diverse patient populations, regulatory advancements, and cost-effectiveness as significant advantages for researchers.
Evidence supporting this claim includes:
- A rise in study registrations
- Successful partnerships
- The region's ability to offer valuable insights into treatment effects across various demographics
These factors collectively position Latin America as an attractive option for medical research.
Introduction
Latin America is emerging as a vibrant epicenter for clinical trials, characterized by diverse patient populations and evolving healthcare landscapes across countries such as Brazil, Mexico, Argentina, and Colombia.
Brazil is projected to lead the region with nearly 10,000 registered clinical studies by 2025, highlighting the unique advantages that the region offers.
This article delves into the dynamic growth of clinical trials in Latin America, exploring the key drivers behind this trend, the challenges faced by researchers, and the regulatory and ethical considerations that shape the research environment.
As the landscape continues to evolve, it presents unparalleled opportunities for innovative medical research, establishing Latin America as a significant player on the global clinical trial stage.
The Landscape of Clinical Trials in Latin America
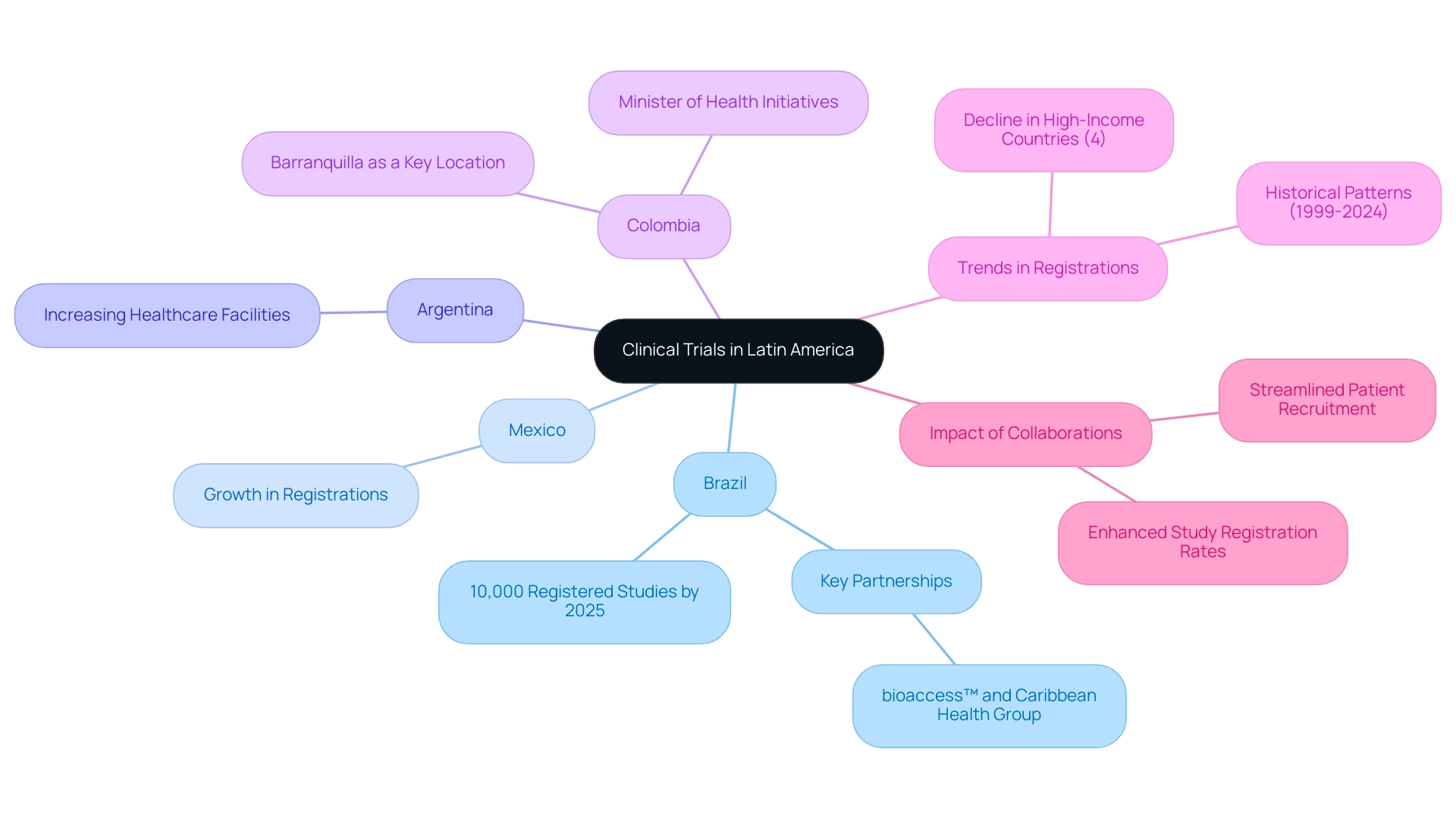
Latin America emerges as a dynamic and diverse region, comprising countries such as Brazil, Mexico, Argentina, and Colombia, each defined by unique healthcare systems and regulatory frameworks. This region has gained prominence as a significant hub for medical trials, driven by its substantial and diverse patient populations, alongside an increasing number of healthcare facilities ready to conduct thorough investigations. By 2025, Brazil is anticipated to lead the region with nearly 10,000 registered studies, underscoring its vital role in the field of medical research.
The rich diversity within Latin America's medical trial regions not only enhances recruitment capabilities but also fosters a comprehensive understanding of treatment effects across various ethnic groups, positioning it as an ideal environment for innovative medical investigations. The landscape for research studies in Latin America is rapidly evolving, with notable growth observed in countries like Mexico, Argentina, and Colombia. Recent statistics reveal a significant rise in registrations across these nations, reflecting their commitment to advancing medical research.
Crucial partnerships, such as that between bioaccess™ and Caribbean Health Group, are establishing Barranquilla as a key location for research studies, supported by initiatives from Colombia's Minister of Health. This expansion is particularly noteworthy given the 4% decline in study registrations experienced by high-income nations between 2020 and 2023, emphasizing the increasing importance of Latin America as a central research hub. Successful medical research examples from these nations, including collaborations with GlobalCare Clinical Trials, illustrate their capacity to deliver valuable insights to the global medical community, achieving over a 50% reduction in recruitment times and 95% retention rates.
Furthermore, findings from the case study titled 'Trends in Clinical Trials Registration (1999-2024)' highlight historical patterns in registrations, emphasizing the evolving role of Latin America in the context of medical studies. As the region continues to enhance its medical study infrastructure, it presents a unique opportunity for scholars to engage with diverse populations and leverage the distinct advantages offered by Latin America. The Horizon Databook, featuring over 1 million market statistics and 20,000+ reports, further emphasizes the critical role of data in decision-making for researchers navigating this changing landscape.
Importantly, the collaboration with Caribbean Health Group is expected to significantly enhance study registration rates and streamline patient recruitment processes, thereby solidifying Barranquilla's position in the research arena.
Key Drivers of Growth in Latin American Clinical Trials
Several key drivers are propelling the growth of clinical trials in Latin America:
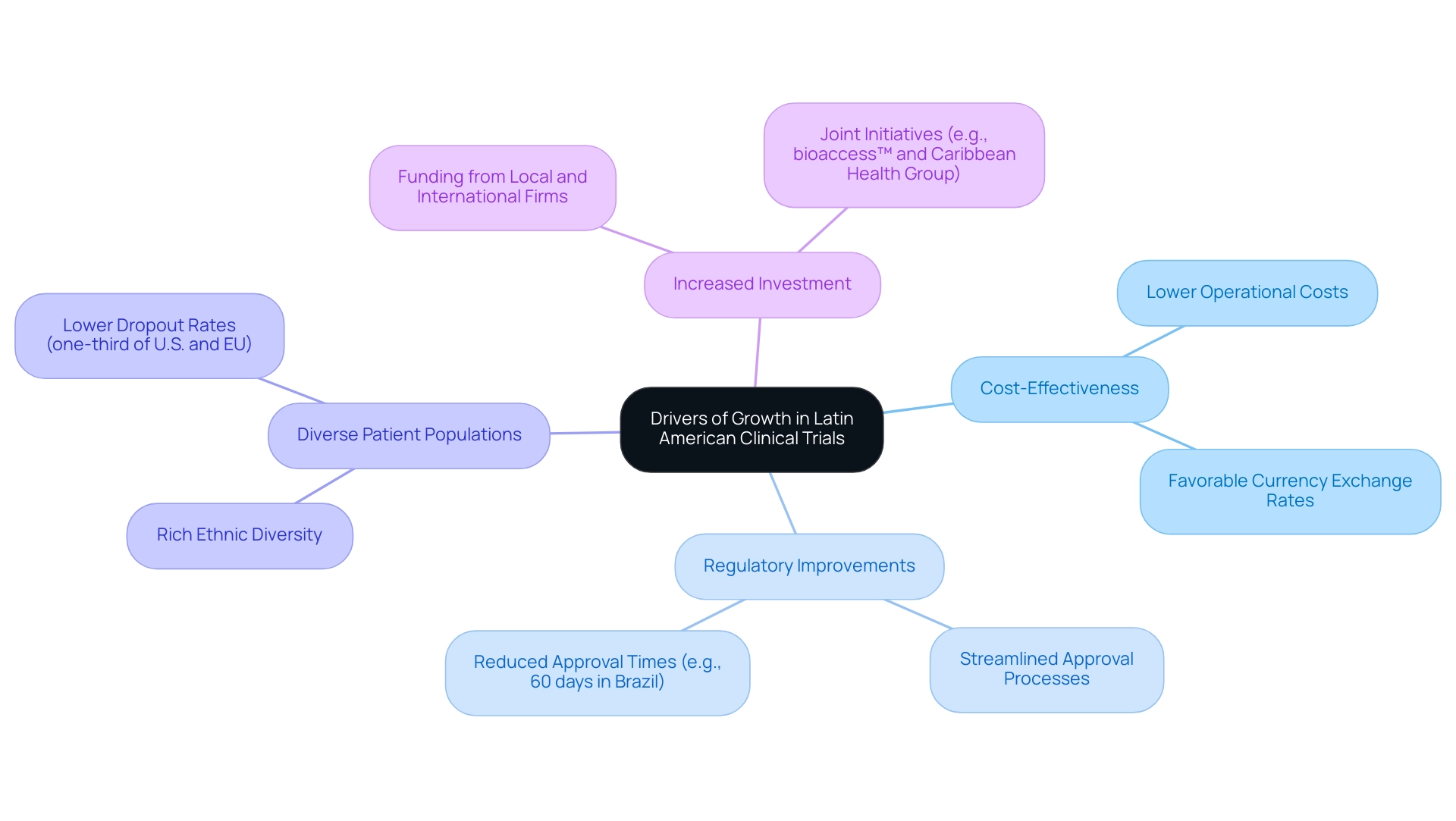
- Cost-Effectiveness: Conducting experiments in Latin America is often significantly more economical than in North America or Europe. This is attributed to lower operational costs and favorable currency exchange rates, creating a financially appealing environment for research.
- Regulatory Improvements: Recent reforms in countries such as Brazil and Mexico have streamlined approval processes, reducing bureaucratic hurdles. For instance, legislative changes in Brazil have cut approval times to as little as 60 days, allowing quicker commencement of evaluations and faster market entry for medical devices. This aligns with the expertise of bioaccess®, which offers expedited medical device research services, ensuring effective study setups and compliance evaluations.
- Diverse Patient Populations: The region's rich ethnic diversity enhances the ability to collect comprehensive data, essential for evaluating the efficacy of treatments across various demographics. This diversity not only enhances the data pool but also ensures that findings are more widely applicable. Moreover, dropout rates in Latin nations are one-third of those in the U.S. and the EU, further emphasizing the reliability of conducting trials in the region.
- Increased Investment: There is a notable rise in funding from both local and international pharmaceutical firms, motivated by the acknowledgment of Latin America's potential for medical studies. This influx of capital is promoting innovation and enhancing the capabilities of trial organizations (CROs) in the region. Joint initiatives, such as those between bioaccess™ and Caribbean Health Group to establish Barranquilla as a top location for medical studies, illustrate the increasing emphasis on improving the area's investigative environment.
These factors collectively position the Latin America medical trial regions as a growing center for research studies, offering unique benefits that are increasingly attractive to researchers and sponsors alike. Furthermore, ongoing regulatory harmonization efforts are progressing, positioning Latin America medical trial regions as significant participants in global research trials. With over 20 years of experience in Medtech, bioaccess® is well-equipped to navigate these complexities, offering extensive research management services, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting.
The case study titled "Accelerated Timelines for Clinical Trials" illustrates how CROs in South America, particularly bioaccess®, are known for their ability to conduct studies quickly due to lower regulatory hurdles and effective patient recruitment strategies. This ultimately contributes to job creation and economic growth in the region.
Challenges in Conducting Clinical Trials in Latin America
Conducting medical studies in Latin America’s trial regions presents substantial advantages, particularly through strategic collaborations and a strong focus on regulatory compliance. Yet, it is imperative to navigate the accompanying challenges effectively. Key issues include:
- Regulatory Complexity: The regulatory landscape varies significantly across countries, with each nation operating under its own framework. This diversity can create a cumbersome and time-consuming process for researchers, complicating the approval and execution of medical studies. For instance, Colombia has instituted a rigorous ICH/GCP certification process that hospitals must complete before engaging in research involving pharmaceutical drugs, thereby ensuring high standards of quality and safety. This certification not only bolsters the reliability of research studies but also highlights the intricate regulatory environment that researchers must navigate. As Julio G. Martinez-Clark, CEO of bioaccess®, notes, "Colombia has acknowledged the advantages of research regulation in Latin regions and has developed an ambitious science, technology, and innovation strategy for 2022–2031 to transition towards a knowledge-based economy."
- Infrastructure Gaps: Although major urban centers in Latin America feature advanced healthcare facilities, rural areas frequently encounter significant infrastructure challenges. The scarcity of adequate medical facilities and resources in these regions can hinder the implementation of studies, limiting access to diverse patient populations and impacting the overall quality of investigation.
- Cultural Barriers: Language differences and varying cultural attitudes toward clinical studies can create additional obstacles in patient recruitment and retention. Grasping local perspectives on medical research is crucial for effective engagement with potential participants, as these factors can substantially influence study outcomes.
- Political Instability: Political fluctuations in certain countries can impact the regulatory landscape and the feasibility of conducting experiments. Such instability may result in abrupt changes to regulations or operational challenges, necessitating that researchers remain vigilant about the political climate in their target regions.
Recent partnerships underscore the potential of Latin America's medical trial regions as pivotal locations for medical studies. For example, bioaccess™ has joined forces with Caribbean Health Group to position Barranquilla as a leading center for research in Latin America’s medical trial regions, with support from Colombia's Minister of Health. This initiative aims to streamline the research process while ensuring that high standards of quality and safety are maintained.
Moreover, collaborations such as that of GlobalCare Clinical Trials with bioaccess™ are focused on enhancing ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and 95% retention rates. As the 2025 DIA Latin meeting approaches in Buenos Aires, discussions surrounding collaboration, alliances, and regulatory practices will be vital for addressing these challenges and advancing the medical research landscape in the region. Incorporating insights from local stakeholders and testimonials will further solidify the credibility of these initiatives and provide a clearer understanding of the operational environment.
Regulatory and Ethical Considerations in Latin American Trials
Conducting clinical studies in Latin America, particularly in Colombia, necessitates strict adherence to a variety of regulatory and ethical standards, which are essential for ensuring the integrity of the study process. Key considerations include:
- Ethics Committees: Approval from local ethics committees is a prerequisite for all trials. In Colombia, these committees are instrumental in assessing the ethical implications of studies, ensuring that participant welfare remains paramount. Hospitals engaged in clinical trials must undergo a rigorous ICH/GCP certification process, which upholds high ethical standards across the region.
- Informed Consent: It is crucial for researchers to facilitate a thorough understanding of the study's purpose, risks, and benefits among participants. This often necessitates translating consent forms into local languages to ensure clarity and comprehension. The challenge of informed consent is particularly pronounced in diverse linguistic and cultural contexts, thereby requiring meticulous attention to communication strategies.
- Data Protection: Adhering to data protection laws is vital, especially regarding the management of personal health information. Researchers must adeptly navigate the complexities of local regulations to protect participant data, an increasingly critical aspect in today's data-driven research landscape.
- Local Regulations: Each Latin American medical trial region possesses its own regulatory framework governing research studies, which can vary significantly from one country to another. In Colombia, the total IRB/EC and MoH (INVIMA) review process typically takes only 90-120 days, substantially expediting the initiation of trials. Furthermore, investments in development and innovation are met with significant financial incentives, including a 100% tax deduction, a 25% tax discount, and a 50% future tax credit, rendering Colombia an attractive destination for medical device companies. With a population exceeding 50 million and approximately 95% coverage under universal healthcare, patient recruitment is notably enhanced, ensuring a diverse participant pool.
The collaboration among local stakeholders and a commitment to regulatory excellence will be pivotal in unlocking the region's potential for trials. In summary, navigating the regulatory and ethical landscape of research in Latin America requires a comprehensive understanding of local guidelines, the proactive involvement of ethics committees, and a steadfast commitment to informed consent and data protection. These elements are essential for executing successful and ethically sound studies in the region, particularly in Colombia, where the combination of cost efficiency, regulatory agility, and high-quality healthcare presents unique opportunities for such investigations.
Best Practices for Successful Clinical Trials in Latin America
To ensure successful medical studies in Latin America, researchers should adopt the following best practices:
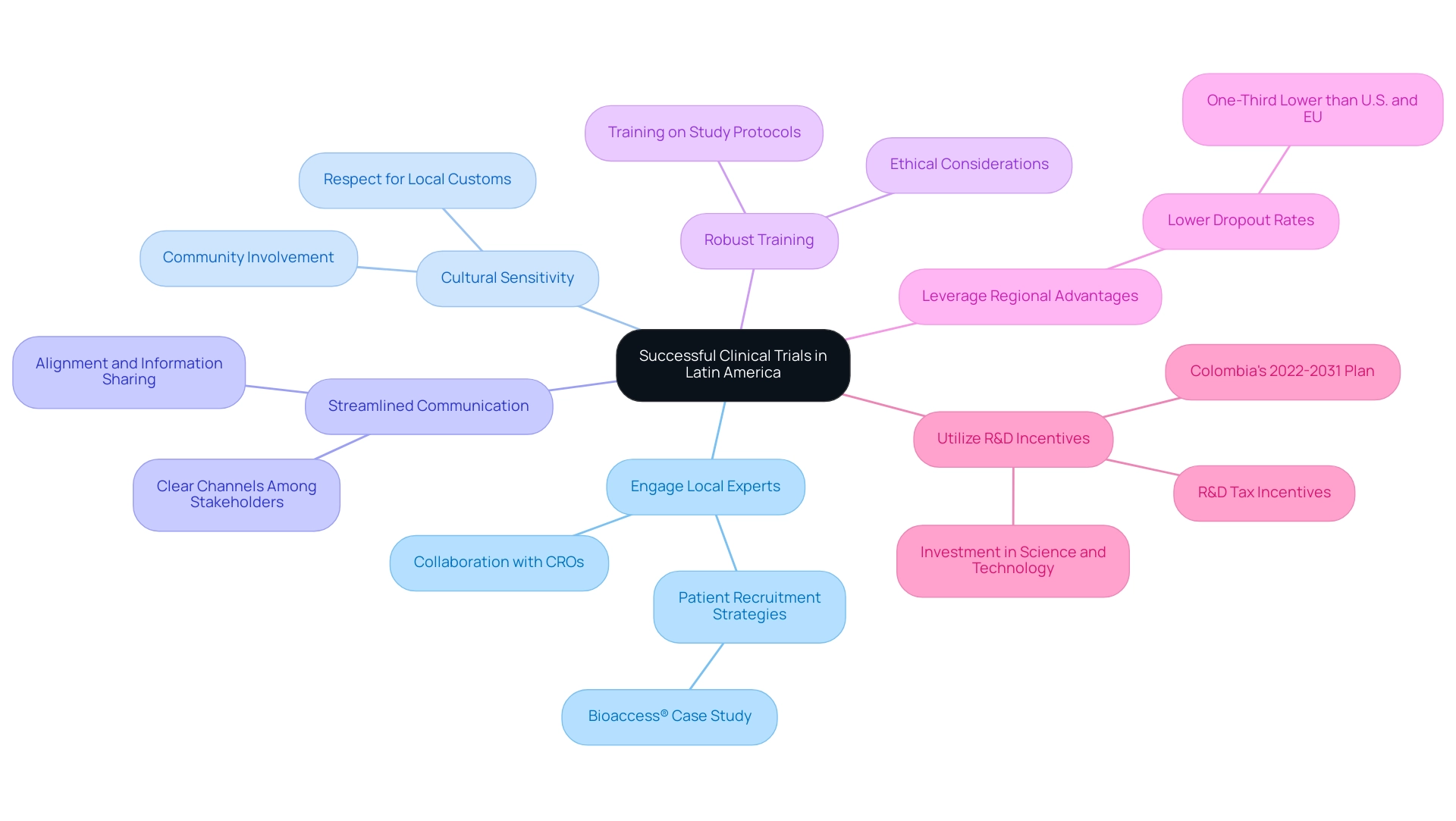
- Engage Local Experts: Collaborating with local research organizations (CROs) is crucial. These experts, such as those at bioaccess®, possess in-depth knowledge of the regulatory landscape and can offer effective patient recruitment strategies tailored to the region. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, highlights how bioaccess® facilitated their first human study in Colombia, emphasizing the importance of local expertise.
- Cultural Sensitivity: Acknowledging and respecting local customs and beliefs significantly enhances participant engagement and retention. This cultural awareness fosters trust and encourages community involvement in research studies.
- Streamlined Communication: Establishing clear and efficient communication channels among all stakeholders—including sponsors, investigators, and participants—is vital for the success of clinical studies. This ensures that everyone is aligned and informed throughout the study process.
- Robust Training: Providing comprehensive training for local staff on study protocols and ethical considerations is essential. This not only improves compliance but also enhances data quality, leading to more reliable outcomes.
- Leverage Regional Advantages: Latin regions boast lower dropout rates compared to the U.S. and EU, with experts noting that these rates are approximately one-third lower. This statistic highlights the region's potential for successful completion of the study.
- Utilize R&D Incentives: Countries like Colombia are actively investing in science, technology, and innovation, supported by R&D tax incentives. Julio G. Martinez-Clark, CEO, noted the country's ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy. These initiatives improve the region's appeal for performing medical research, establishing it as a strategic option for Medtech firms.
By applying these best practices and utilizing the knowledge of organizations like bioaccess®, which possesses over 20 years of experience in Medtech, researchers can manage the intricacies of medical studies in Latin America efficiently. Furthermore, bioaccess® offers comprehensive clinical study management services, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies. Additionally, understanding the regulatory framework established by INVIMA, Colombia's National Food and Drug Surveillance Institute, is crucial for ensuring compliance and successful execution.
These factors reinforce Latin's status as a Medtech hub.
Future Trends and Opportunities in Latin American Clinical Trials
The future of clinical trials in Latin America is marked by several promising trends that are reshaping the landscape:
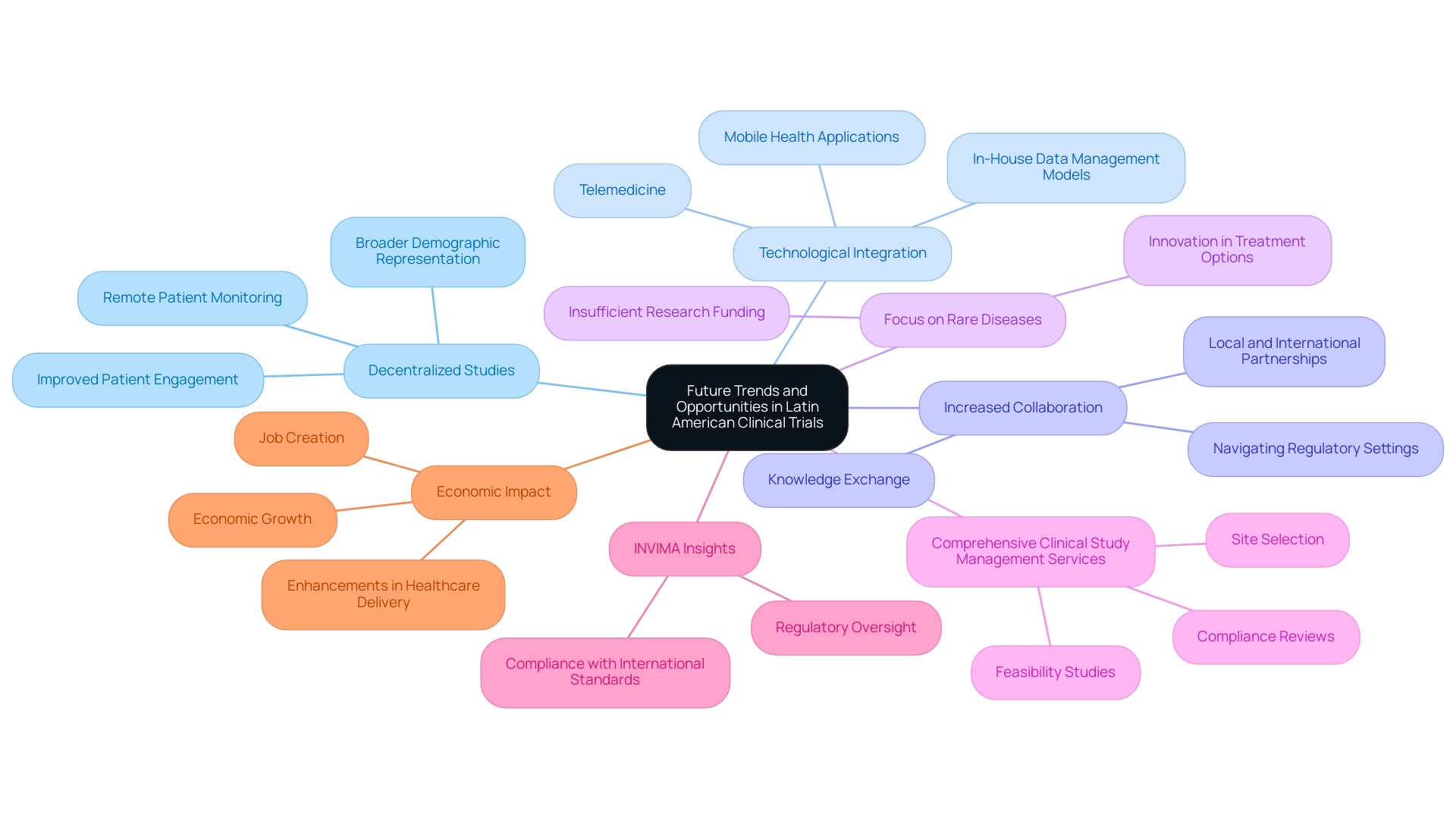
- Decentralized Studies: The adoption of decentralized research studies (DCTs) is transforming how investigations are conducted. By enabling remote patient monitoring and data collection, DCTs significantly enhance accessibility for participants, allowing for broader demographic representation and improved patient engagement. Bioaccess’s project management capabilities ensure that these assessments are executed efficiently, maintaining high standards of oversight.
- Technological Integration: The integration of advanced technologies, including telemedicine and mobile health applications, is revolutionizing patient engagement and data collection processes. These innovations not only optimize operations but also facilitate real-time data access, essential for prompt decision-making in medical studies. A notable trend among sponsors is the shift towards in-house data management models, moving away from reliance on CROs to enhance control over data and improve study operationalization. Bioaccess supports this transition with comprehensive monitoring services that guarantee compliance and data integrity.
- Increased Collaboration: There is a notable trend towards increased collaboration between local and international organizations, fostering knowledge exchange and resource sharing. These collaborations improve the quality and efficiency of research studies and are crucial for navigating intricate regulatory settings. Addressing linguistic and cultural differences is vital for reconciling local customs with ethical guidelines in clinical studies.
- Focus on Rare Diseases: A growing interest in conducting studies for rare diseases is emerging, driven by the recognition that these conditions often receive insufficient research funding and attention. This shift not only addresses unmet medical needs but also opens new avenues for innovation in treatment options.
- Comprehensive Clinical Study Management Services: Companies like Bioaccess excel in offering a range of services, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting. This thorough approach guarantees that evaluations are performed effectively and in accordance with local regulations, with a strong focus on project management and oversight to adapt to the changing environment of research studies.
- INVIMA Insights: The Colombia National Food and Drug Surveillance Institute (INVIMA) plays a critical role as a Level 4 health authority by PAHO/WHO, overseeing medical device regulation and ensuring compliance with international standards. This regulatory oversight is vital for the success of research trials in the region, as it helps navigate complex approval processes.
- Economic Impact: The influence of Medtech research studies on local economies is significant, contributing to job creation, economic growth, and enhancements in healthcare delivery. These studies promote international cooperation, further enhancing the region's research capabilities.
As the worldwide market for precision oncology is anticipated to reach $98 billion by 2025, the opportunity for expansion in medical studies within the Latin American medical trial regions is considerable. Furthermore, with approximately 30% of the population under 14 years old, there is a burgeoning elderly patient demographic that presents a significant opportunity for new drug markets.
In summary, the landscape of medical studies in Latin America is evolving swiftly, with decentralized studies and technological progress taking the lead. These trends not only promise to improve the efficiency and effectiveness of medical research but also establish significant Latin American medical trial regions as participants in the global research arena. As noted by Dipanwita Das, "Last, but certainly not the least is regulatory preparedness. Regulations are becoming increasingly intricate, prescriptive, and demanding, but monitoring FDA guidance on novel study designs and DCT approaches, remaining up-to-date with international regulations, ensures a very smooth commercialization process, along with data privacy.
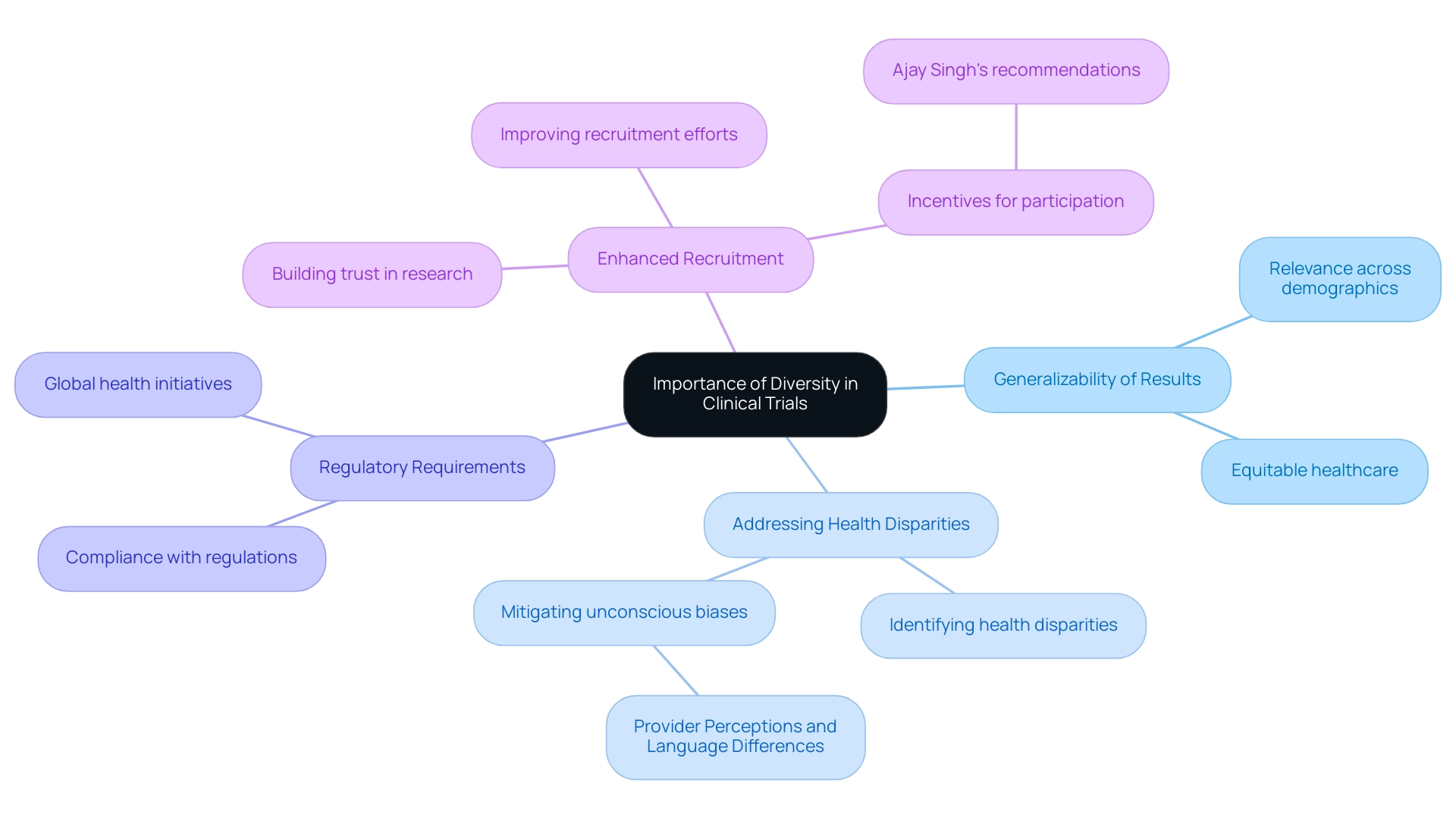
The Importance of Diversity in Latin American Clinical Trials
Diversity in clinical trials is essential for multiple reasons:
- Generalizability of Results: Including diverse populations in medical studies significantly enhances the relevance of findings across various patient demographics. This is particularly vital in 2025, as the medical community increasingly acknowledges that treatments must be effective for all groups to ensure equitable healthcare. With over 15 years of experience in the Medtech sector, bioaccess® is strategically positioned to foster inclusivity in research studies, particularly through its partnership with Caribbean Health Group, which aspires to position Barranquilla as a premier location for medical investigations in Latin America. This initiative receives further backing from Colombia's Minister of Health, Juan Pablo Uribe, who attended the collaboration announcement meeting on March 29, 2019, in Miami, FL.
- Addressing Health Disparities: Trials that reflect the demographic composition of the population can effectively identify and mitigate health disparities. For instance, studies conducted in Latin America have revealed how unconscious biases among healthcare professionals can lead to differential treatment outcomes for minority patients. The case study titled "Provider Perceptions and Language Differences" illustrates that these biases, coupled with language barriers, complicate communication and understanding of medical conditions. By incorporating these groups in research studies, investigators can gain deeper insights and address these inequalities.
- Regulatory Requirements: Regulatory agencies are increasingly emphasizing the necessity of diversity in research trials. This shift aims to ensure that new medical treatments are safe and effective for all demographic groups, aligning with global health initiatives advocating for inclusive study practices. bioaccess® is committed to navigating these regulatory landscapes, ensuring compliance while promoting diverse participation in clinical studies.
- Enhanced Recruitment: Engaging with diverse communities not only improves recruitment efforts but also fosters trust in the research process. Successful experiments, such as the SPRINT and ADAPT studies, demonstrate that diverse participant pools can lead to significant improvements in outcomes and safety. Moreover, as highlighted by Ajay Singh, senior associate dean for postgraduate medical education at Harvard Medical School, offering suitable incentives for potential study participants can enhance a more varied recruitment strategy. With bioaccess®'s comprehensive research study management services, including feasibility studies and project management, recruitment efforts are streamlined to encompass a broader demographic.
In summary, the significance of diversity in medical studies cannot be overstated. It is crucial for ensuring that research findings are relevant and beneficial to all segments of the population, ultimately leading to improved health outcomes and more effective medical interventions. By leveraging its expertise and customized approach, bioaccess® aims to advance medical devices more swiftly, ensuring that clinical trials in Latin America reflect the diversity of the populations they serve, benefiting local economies and enhancing healthcare outcomes.
Conclusion
The growth of clinical trials in Latin America underscores the region's unique advantages, such as diverse patient populations and evolving regulatory frameworks. Countries like Brazil, Mexico, Argentina, and Colombia are not only witnessing an increase in trial registrations but are also emerging as significant players on the global clinical research stage. The anticipated rise in Brazil's registered clinical studies by 2025 exemplifies the potential for innovation and presents an opportunity for researchers to engage with a rich and varied demographic landscape.
However, while the prospects are promising, challenges such as regulatory complexity, infrastructure gaps, and cultural barriers must be navigated effectively. Collaborations among local and international organizations, exemplified by initiatives in Barranquilla, are crucial for overcoming these obstacles and enhancing the clinical research environment. Emphasizing ethical considerations, including informed consent and data protection, is equally vital to uphold the integrity of the research process.
Looking ahead, the trends towards decentralized trials and technological integration signal a transformative shift in how clinical research is conducted in the region. These advancements not only facilitate broader participation but also promise to enhance the efficiency and effectiveness of trials. Ultimately, the commitment to diversity in clinical trials will lead to more equitable healthcare solutions, addressing health disparities and ensuring that new treatments are safe and effective for all demographic groups.
As Latin America continues to establish itself as a hub for clinical trials, it presents an unparalleled opportunity for innovative medical research. With the right strategies and partnerships in place, the region is poised to make significant contributions to the global medical community, enhancing health outcomes and advancing scientific knowledge.
Frequently Asked Questions
What countries are included in Latin America as key players in medical trials?
Latin America includes countries such as Brazil, Mexico, Argentina, and Colombia, each with unique healthcare systems and regulatory frameworks.
Why has Latin America become a significant hub for medical trials?
Latin America has gained prominence as a hub for medical trials due to its substantial and diverse patient populations, an increasing number of healthcare facilities, and the ability to conduct thorough investigations.
What is the projected number of registered studies in Brazil by 2025?
By 2025, Brazil is anticipated to lead the region with nearly 10,000 registered studies.
How does the diversity within Latin America benefit medical trials?
The rich diversity enhances recruitment capabilities and fosters a comprehensive understanding of treatment effects across various ethnic groups, making it an ideal environment for innovative medical investigations.
What recent trends have been observed in clinical trial registrations in Latin America?
There has been significant growth in study registrations in countries like Mexico, Argentina, and Colombia, reflecting their commitment to advancing medical research.
What partnerships are contributing to the growth of medical studies in Latin America?
Partnerships, such as between bioaccess™ and Caribbean Health Group, are establishing Barranquilla as a key location for research studies, supported by initiatives from Colombia's Minister of Health.
How does the decline in study registrations in high-income nations affect Latin America?
The 4% decline in study registrations in high-income nations between 2020 and 2023 emphasizes the increasing importance of Latin America as a central research hub.
What are some advantages of conducting clinical trials in Latin America?
Advantages include cost-effectiveness, regulatory improvements, diverse patient populations, and increased investment from pharmaceutical firms.
How have regulatory reforms impacted clinical trials in Latin America?
Recent reforms have streamlined approval processes, significantly reducing bureaucratic hurdles, such as Brazil's legislative changes that have cut approval times to as little as 60 days.
What is the significance of the case study 'Accelerated Timelines for Clinical Trials'?
This case study illustrates how CROs in South America, particularly bioaccess®, are known for their ability to conduct studies quickly due to lower regulatory hurdles and effective patient recruitment strategies.
What role does bioaccess® play in the Latin American clinical trial landscape?
Bioaccess® offers extensive research management services, including feasibility studies, site selection, compliance reviews, study setup, and project management, leveraging over 20 years of experience in Medtech.




