Overview
The title "9 Ways to Drive Medtech Innovation Through Ecuadorian Research" poses a critical question: how can we enhance medical technology innovation in Ecuador? This article delineates various strategies, including:
- Leveraging Ecuador's diverse patient population
- Establishing strategic collaborations with local institutions
- Utilizing a supportive regulatory environment
Each of these approaches fosters a conducive landscape for medical technology advancements and research efficiency, underscoring the importance of collaboration in driving innovation within the Medtech sector.
Introduction
Ecuador is rapidly emerging as a beacon of innovation in the Medtech sector, driven by a unique combination of favorable regulatory frameworks, diverse patient populations, and cost-effective research opportunities. As the demand for advanced medical technologies rises, organizations like bioaccess® stand at the forefront, providing invaluable clinical research services that bridge the gap between groundbreaking medical devices and market readiness.
With over 15 years of expertise, bioaccess® not only navigates the complexities of clinical trials but also fosters strong local partnerships that enhance data quality and drive ethical research practices. This article delves into the myriad factors that position Ecuador as a pivotal player in Medtech innovation, exploring how its strategic advantages can unlock new opportunities for companies eager to make a significant impact in the healthcare landscape.
bioaccess: Leading Clinical Research Organization Driving Medtech Innovation in Ecuador
bioaccess® stands out as a premier contract development organization (CRO) in Latin America, dedicated to delivering specialized clinical services tailored for the medical technology sector. With over 15 years of expertise, bioaccess® adeptly navigates the complexities involved in transitioning medical devices from initial studies to market readiness, all while ensuring strict adherence to regulatory standards. Their comprehensive process includes:
- Feasibility assessments
- Study location selection
- Investigator recruitment
- Regulatory compliance
- Project management
- Meticulous reporting
This positions bioaccess® as an indispensable partner for medical technology startups eager to innovate and excel within Ecuador's dynamic healthcare landscape, promoting medtech innovation through Ecuadorian research that is increasingly recognized for its favorable regulatory environment, often regarded as a model for other Latin American countries. This growing reputation is underscored by the rising number of U.S. medical technology firms seeking dependable data and efficient patient recruitment in the region. By fostering robust connections with local researchers, bioaccess® enhances study success rates, ensuring that innovative medical devices can reach the market more swiftly and effectively.
The organization’s commitment to providing tailored consulting services further solidifies its leadership in the research arena. As Ecuador emerges as a hub for Medtech innovation through Ecuadorian research, bioaccess® remains at the forefront, driving advancements aimed at improving healthcare outcomes and enriching patient lives throughout the region. Notably, Colombia's evolving image in clinical trials serves as a comparative backdrop, illustrating how neighboring nations have successfully attracted U.S. medical technology firms, thereby highlighting Ecuador's potential as a center for medical technology. As emphasized by marketing expert Nick Tippmann, the strategic contributions of CROs like bioaccess® are vital for fostering innovation in the medical technology sector. Ecuador is poised to become a significant player in the Medtech landscape, particularly with its focus on Medtech innovation through Ecuadorian research, which is increasingly viewed as a benchmark for other Latin American countries.
Diverse Patient Population: Enhancing Data Quality for Clinical Trials
Ecuador's diverse demographic fabric presents a significant opportunity for research studies, particularly through the comprehensive research management services offered by bioaccess®. By incorporating participants from various ethnic backgrounds, researchers can gain a nuanced understanding of the efficacy and safety of medical devices across diverse populations. This inclusivity not only enhances data quality but also ensures that findings are applicable to a broader audience, ultimately improving patient care and outcomes.
The impact of ethnic diversity on research data quality is profound. Diverse patient populations can reveal variations in treatment responses, which are crucial for developing effective medical devices. For example, a study analyzing COVID-19 data in Ecuador highlighted how socio-demographic factors influenced health outcomes, underscoring the importance of considering diverse backgrounds in medical research. Understanding the epidemiological patterns of COVID-19 in Latin America further emphasizes the significance of this diversity in medical studies.
In 2025, Ecuador's demographic statistics indicate a continuing trend toward greater ethnic diversity, with notable representation from Indigenous communities and other cultural groups. This variety is essential for research studies, as it facilitates a thorough evaluation of medical devices in practical environments. By leveraging this demographic diversity, researchers can ensure that studies yield outcomes that are not only statistically significant but also practically relevant across various populations.
Case studies, such as those examining cultural influences in Ecuador, further illustrate the importance of understanding local contexts in research. These studies reveal how cultural practices and beliefs can affect patient involvement and compliance, ultimately influencing study outcomes. Additionally, the approach to data collection and analysis, as demonstrated in research involving COVID-19 patients, highlights the critical role of socio-demographic information in understanding health effects and improving trial results.
As Lila Adana from the Universidad de Las Americas notes, "Integrating varied viewpoints in medical research is crucial for creating solutions that are effective across different populations." With over 20 years of experience in medical technology, bioaccess® is well-equipped to navigate these complexities. As the medical technology sector continues to evolve, embracing Ecuador's diverse patient demographic will be vital for fostering Medtech innovation through Ecuadorian research and enhancing healthcare solutions, particularly through the expedited medical device research services provided by bioaccess®, which include:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Research Follow-Up Studies
Cost-Effective Research Opportunities: Maximizing Budgets for Medtech Startups
Carrying out medical studies in Ecuador presents significant financial advantages compared to North America and Europe. The notably reduced operational expenses enable Medtech startups to optimize their funding for studies, leading to Medtech innovation through Ecuadorian research, facilitating more comprehensive investigations or the capacity to conduct several tests simultaneously. This financial flexibility is crucial for startups striving to innovate without the burden of excessive financial strain. In fact, studies in Ecuador can be up to 50% cheaper than those in North America, making it an increasingly appealing location for research.
Furthermore, bioaccess® provides comprehensive clinical trial management services, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
Ecuador's regulatory agencies are open to collaboration with international organizations, which greatly benefits Medtech innovation through Ecuadorian research by providing essential guidance for navigating regulatory processes and ensuring compliance with global standards. This encouraging atmosphere, along with sustainable funding systems that extend beyond initial project life spans, cultivates a setting where continuous investigative initiatives can thrive. Ultimately, these elements benefit both researchers and patients alike, showcasing the importance of Medtech innovation through Ecuadorian research.
Moreover, insights gained from Ecuador's experience during the Revolución Ciudadana highlight the significance of robust governance and coordinated health research, further reinforcing the advantages of conducting medical studies in this region.
Supportive Regulatory Environment: Streamlining Clinical Trial Approvals
Ecuador has established a robust regulatory framework that significantly accelerates the approval of clinical trials. Recent reforms have streamlined the application process, effectively reducing bureaucratic barriers and shortening timelines for medical technology firms. This proactive stance by regulatory authorities not only stimulates Medtech innovation through Ecuadorian research but also attracts international research initiatives, positioning Ecuador as a formidable competitor in the global Medtech arena.
In 2025, the nation experienced a significant rise in research approvals, indicating the beneficial impact of these regulatory changes. The introduction of the Portal of Medical Studies of the Americas at the end of 2024 further enhances research in the region, increasing Ecuador's appeal.
Furthermore, as Julio G. Martinez-Clark notes, "Ecuador offers a compelling value proposition for researchers seeking to maximize their budgets without compromising quality." The focus on ethical adherence and community involvement has positioned Ecuador as a reliable and credible location for studies, attracting organizations that prioritize integrity and inclusiveness in their work.
bioaccess® exemplifies this commitment through its comprehensive trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
Additionally, bioaccess® adheres to strict grievance and data protection procedures, ensuring that client concerns are addressed with compliance and transparency. A case study on ethical considerations emphasizes how Ecuador mandates that researchers adhere to strict ethical guidelines to safeguard participants' rights, privacy, and well-being, fostering trust and ensuring that studies address local needs.
By nurturing enduring partnerships through teamwork, Ecuador fosters Medtech innovation through Ecuadorian research, creating pathways for future medical exploration opportunities and enhancing its standing as a center for medical advancements.
High-Quality Healthcare Infrastructure: Ensuring Reliable Clinical Trials
Ecuador's healthcare system is notably advanced, featuring modern hospitals and specialized clinics that are adeptly equipped to conduct research studies. This extensive network plays a vital role in participant recruitment, treatment delivery, and data collection—critical components that uphold the integrity of medical studies. With a total of 769 health facilities across the nation, the presence of skilled healthcare professionals significantly bolsters the reliability of study outcomes. This combination of resources positions Ecuador as an ideal hub for Medtech innovation through Ecuadorian research, which fosters innovation and ensures high-quality research results. In this context, bioaccess® emerges as a leading contract research organization, facilitating medical device studies throughout Latin America. Its partnerships, including one with GlobalCare Clinical Trials, have demonstrated remarkable success, achieving over a 50% reduction in subject recruitment time and a retention rate exceeding 95%. These outcomes highlight bioaccess's essential role in enhancing ambulatory services for research in Colombia and beyond, suggesting the potential for similar advancements in Ecuador.
Nevertheless, recent case studies have unveiled significant shortcomings in research reporting, with merely 24 out of 75 identified studies found in the national registry. This lack of compliance raises serious concerns regarding the integrity of research data and underscores the urgent need for improved oversight and adherence to established practices. As Ecuador continues to enhance its medical study capabilities, it must embrace medtech innovation through Ecuadorian research to adapt to the evolving demands of the medical technology landscape. The engagement of proficient healthcare experts not only facilitates the execution of studies but also ensures that the research conducted meets the highest standards of quality, further solidifying Ecuador's role as a key player in the Medtech sector.
Strategic Location: Enhancing Logistics for Medtech Research
Ecuador's strategic location in the heart of South America offers substantial logistical advantages for conducting clinical studies. Its proximity to various Latin American countries simplifies participant recruitment, enabling a diverse demographic that enhances the representativeness of trial data. This diversity is crucial for generating insights that are relevant across different populations, ultimately bolstering the validity of findings.
Moreover, Ecuador boasts a robust transportation system that facilitates the movement of study materials and personnel. Efficient logistics are vital for maintaining the integrity of medical studies, ensuring timely delivery of equipment and supplies, as well as the swift mobilization of research teams. The nation's infrastructure meets these operational needs, making it an attractive site for biotechnology firms like bioaccess®, which specialize in comprehensive research management services, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Research Follow-Up Studies (PMCF)
With over 20 years of experience in Medtech, bioaccess® possesses extensive knowledge and expertise to navigate the complexities of clinical trials.
In addition to these logistical strengths, Ecuador's health study output has seen a significant increase, particularly following the implementation of policies aimed at enhancing study capacity. Nonetheless, there remains an urgent need for better alignment of investigative initiatives with national health priorities, as highlighted in recent analyses. This ongoing development in the study environment presents an opportunity for medical technology companies to leverage local insights and address these challenges. As Marisa Piaggio, Clinical Project Manager Latin America, remarked, "I hope these few tips would help you to avoid one or two bumps in the road and maybe save you some time and resources."
In conclusion, the logistical benefits of conducting medical studies in Ecuador, combined with its strategic location and the expertise of bioaccess®, position the nation as a promising hub for Medtech innovation through Ecuadorian research and development. The longitudinal analysis of health studies in Ecuador confirms a significant increase in health study output post-2008, further underscoring the potential for medical technology trials in this evolving landscape. Additionally, the impact of medical technology studies on local economies, such as job creation and healthcare improvements, highlights the broader benefits of investing in clinical investigations in this region.
Collaboration Opportunities: Partnering with Local Institutions for Enhanced Research
Ecuador offers a significant array of collaboration opportunities for Medtech firms looking to drive Medtech innovation through Ecuadorian research and enhance their development capabilities. By forming partnerships with local universities, research institutions, and healthcare providers, these companies can promote medtech innovation through Ecuadorian research while leveraging invaluable local expertise, accessing state-of-the-art facilities, and engaging with diverse patient populations. Such collaborations not only facilitate knowledge exchange but also drive Medtech innovation through Ecuadorian research, resulting in more efficient research studies and improved healthcare solutions.
The influence of these partnerships is clearly reflected in the burgeoning medical devices market in Ecuador, which thrives on stable economic growth and favorable government policies. Macroeconomic factors, particularly increasing healthcare expenditure, have drawn both domestic and international investments into the sector. Case studies reveal that stable economic growth and supportive policies have significantly shaped the medical device market, further emphasizing the critical role of local collaborations in boosting the success rates of clinical trials.
Additionally, partnerships with universities have proven to be especially advantageous. These institutions routinely provide access to cutting-edge development resources, fostering an environment where innovative ideas can flourish. Collaborative initiatives between medical technology firms and nearby universities have led to advancements in healthcare innovation, illustrating the potential for Medtech innovation through Ecuadorian research. For example, initiatives aimed at addressing chronic malnutrition in children under five years, as highlighted by the Ecuadorian National Institute of Statistics and Census (INEC), illustrate the pressing public health challenges that technology solutions can address through local collaborations.
Statistics demonstrate an upward trend in partnerships between medical technology firms and local institutions, signifying a growing acknowledgment of the value these collaborations offer. As Julio Martinez-Clark, CEO of bioaccess™, articulates, "Latin America is an unexplored destination for Medtech studies," urging the implementation of trials and the enhancement of regional opportunities. As the medical technology market in Ecuador continues to expand, influenced by consumer preferences and digitalization trends, the significance of local partnerships will be pivotal in shaping the future of healthcare studies in the region.
Ethical Considerations: Building Trust Through Community Engagement
Ethical considerations are paramount in medical studies, especially within diverse communities like Ecuador. Engaging local communities through transparent communication and active participation fosters trust and encourages involvement in research initiatives. Notably, a study backed by NIH grants revealed that approximately 53% of participants were sourced from the community, many of whom lacked prior experience in medical studies. This underscores the critical importance of inclusivity in research efforts.
Implementing strategies to mitigate logistical barriers—such as transportation, flexible appointment scheduling, and childcare—can significantly enhance recruitment outcomes. For example, case studies demonstrate that offering telehealth options and transportation assistance can substantially increase the likelihood of attracting a diverse participant pool. The case study titled 'Facilitating Access to Clinical Trials' illustrates how addressing these logistical challenges can elevate participation rates.
Furthermore, initiatives like the Health Equity through Diversity Webinar Series emphasize the necessity of community involvement and collaborative solution development to improve participant diversity in studies, addressing medical distrust and other challenges faced by underrepresented groups, which aligns with the goals of Medtech innovation through Ecuadorian research.
By prioritizing ethical standards, Medtech firms ensure that their investigations are conducted responsibly, respecting the rights and well-being of participants while simultaneously enhancing the credibility of their findings. bioaccess's comprehensive research management services—including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting—are instrumental in upholding these ethical practices.
As Wei Kong aptly stated, 'Integrity is doing the right thing, even if nobody is watching.' This commitment to ethical principles ultimately yields more robust and representative research outcomes, paving the way for advancements in Medtech innovation through Ecuadorian research that genuinely reflect the needs of the communities they serve.
Access to Untapped Patient Populations: Unlocking New Research Opportunities
Ecuador presents a distinctive opportunity to engage underrepresented patient populations, particularly within rural and indigenous communities. By incorporating these groups into research studies, the information gathered is enriched, enhancing the understanding of medical devices' effectiveness and safety across diverse demographics. Notably, research indicates that 67.6% of respondents reported chronic conditions, underscoring significant health disparities that persist despite rural residents participating in clinical trials at rates exceeding their population proportion.
Prioritizing these communities allows medical technology firms, such as bioaccess®, to strengthen the validity of their findings and foster more equitable healthcare solutions. Engaging with rural and indigenous populations can yield innovative insights that drive Medtech innovation through Ecuadorian research, ultimately benefiting a broader spectrum of patients.
As González-Andrade, F. pointed out, "It is essential to conduct more studies that involve hospitalized and ambulatory patients to have a clearer picture of the epidemic in the country."
Furthermore, bioaccess® provides extensive clinical trial management services, ensuring compliance with the Helsinki Declaration and applicable national regulations, thus addressing ethical considerations in conducting studies within these communities. This approach not only supports healthcare improvement but also contributes to job creation and economic growth in the region.
Transformative Impact: Why Ecuador is a Game Changer for Medtech Innovation
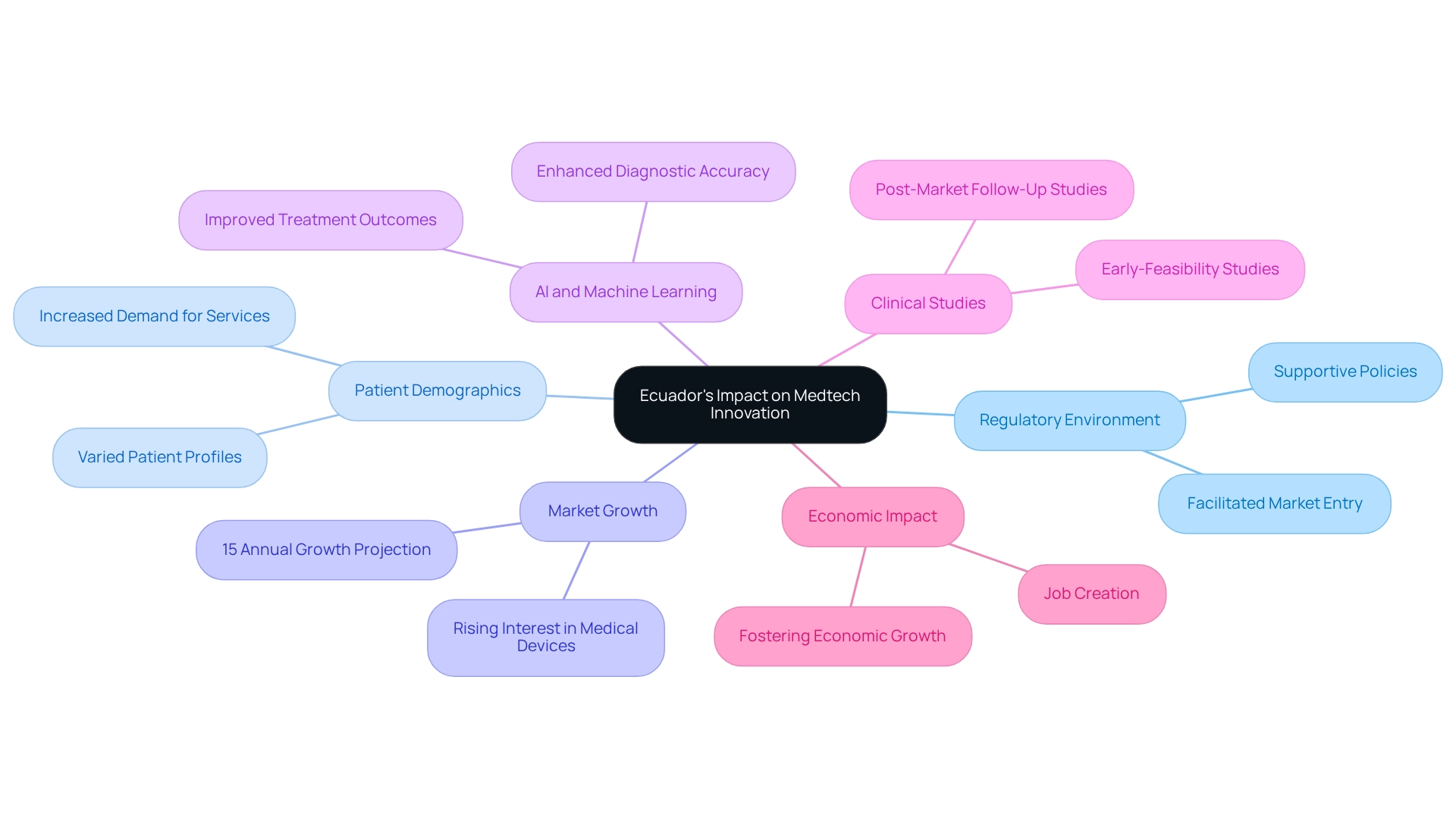
Ecuador's distinct combination of a supportive regulatory environment, varied patient demographics, and affordable research opportunities positions it as a key player in medical technology innovation. The country's healthcare infrastructure is rapidly improving, which is anticipated to further stimulate demand for medical devices. By 2025, the medical technology market in Ecuador is projected to experience significant growth, with estimates indicating an annual increase of approximately 15%. This growth reflects the rising interest in advanced technologies such as artificial intelligence and machine learning, which are enhancing diagnostic accuracy and treatment outcomes. Recent trends indicate that the application of AI in medical technology is gaining traction in Ecuador, aiming to elevate healthcare delivery and patient care.
As Arthur Conan Doyle aptly noted, "It is a capital mistake to theorize before one has data." This underscores the importance of data-driven methodologies in medical technology innovation. By leveraging Ecuador's advantages, medical technology firms can streamline their research and development processes, facilitating quicker market entry and improved patient outcomes. With over 20 years of experience, bioaccess® offers expertise in managing extensive trial services—including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies—enabling companies to navigate the intricacies of trials effectively. The transformative impact of Ecuador's healthcare policies is evident, as they cultivate an environment conducive to innovation, ultimately benefiting the broader medical technology ecosystem. Moreover, medical technology clinical studies contribute to local economies by creating jobs and fostering economic growth. For Directors of Clinical Research, harnessing these insights can enhance strategic planning and decision-making, ensuring that their organizations remain at the forefront of Medtech advancements.
Conclusion
Ecuador is emerging as a key player in Medtech innovation, driven by its favorable regulatory environment, diverse patient populations, and cost-effective clinical research opportunities. Organizations like bioaccess® are crucial in navigating clinical trials, ensuring that medical devices transition smoothly from concept to market. With over 15 years of experience, bioaccess® enhances research quality through local partnerships, making it an invaluable asset for Medtech startups.
The country's demographic diversity significantly enhances data quality, ensuring that medical devices are tested across various populations and ultimately improving healthcare outcomes. Engaging underrepresented communities further fuels innovation and the relevance of research findings.
Moreover, conducting clinical trials in Ecuador offers substantial cost advantages, complemented by a supportive regulatory framework that streamlines approval processes and facilitates ethical research practices. As the Medtech market continues to expand, collaboration with local institutions will be vital in advancing research capabilities and addressing public health challenges.
In conclusion, Ecuador's unique strengths position it as a transformative force in the Medtech sector. By leveraging these advantages, Medtech companies can enhance their research efforts while contributing to economic growth and improved patient care. Embracing Ecuador's potential will foster a more inclusive and effective Medtech landscape, ultimately benefiting patients and communities alike.
Frequently Asked Questions
What is bioaccess® and what services do they provide?
bioaccess® is a contract development organization (CRO) in Latin America that specializes in clinical services for the medical technology sector. They offer services such as feasibility assessments, study location selection, investigator recruitment, regulatory compliance, project management, and meticulous reporting.
How does bioaccess® support medical technology startups?
bioaccess® supports medical technology startups by helping them navigate the complexities of transitioning medical devices from initial studies to market readiness while ensuring compliance with regulatory standards. Their tailored consulting services and strong connections with local researchers enhance study success rates.
Why is Ecuador considered a favorable location for medical research?
Ecuador is seen as a favorable location for medical research due to its diverse demographic, which allows for comprehensive studies across various ethnic backgrounds. This diversity improves data quality and ensures the findings are applicable to a broader audience, ultimately enhancing patient care.
What are the advantages of conducting medical studies in Ecuador compared to North America and Europe?
Conducting medical studies in Ecuador can be up to 50% cheaper than in North America, allowing Medtech startups to optimize their funding for research. The reduced operational expenses enable more comprehensive investigations and the ability to conduct multiple tests simultaneously.
What types of studies does bioaccess® conduct?
bioaccess® conducts various types of studies, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Research Follow-Up Studies.
How does ethnic diversity impact the quality of research data?
Ethnic diversity in research populations can reveal variations in treatment responses, which are crucial for developing effective medical devices. This inclusivity enhances data quality and ensures findings are relevant across different populations.
What role do Ecuador's regulatory agencies play in medical research?
Ecuador's regulatory agencies are open to collaboration with international organizations, providing essential guidance for navigating regulatory processes and ensuring compliance with global standards, which benefits Medtech innovation through Ecuadorian research.
How does bioaccess® contribute to improving healthcare outcomes in the region?
By facilitating Medtech innovation through Ecuadorian research and providing expedited medical device research services, bioaccess® helps drive advancements aimed at improving healthcare outcomes and enriching patient lives throughout the region.




