Overview
The article emphasizes best practices for compliance strategies tailored to clinical trials in Brazil, highlighting the critical need to understand and navigate the regulatory landscape overseen by ANVISA and ethical committees. It underscores the necessity of thorough documentation, local expertise, and ongoing education to effectively address the complexities inherent in Brazil's regulations. This approach not only ensures the ethical conduct of medical research but also enhances its efficiency.
Introduction
Brazil's clinical trial landscape is undergoing a significant transformation, driven by evolving regulations and a heightened focus on compliance. At the forefront of this change is the National Health Surveillance Agency (ANVISA), which oversees the intricate web of guidelines governing clinical research in the country.
Recent legislative advancements, including Law 14,874/2024, have streamlined the approval process for clinical trials. However, the complexities of navigating ethical considerations and regulatory requirements persist. Researchers must remain informed about the latest developments to ensure adherence to Good Clinical Practice (GCP) and safeguard participant rights.
As Brazil continues to emerge as a pivotal hub for clinical trials, understanding the regulatory framework and implementing best practices will be essential for achieving successful outcomes in medical research and innovation.
Navigating Brazil's Regulatory Landscape for Clinical Trials
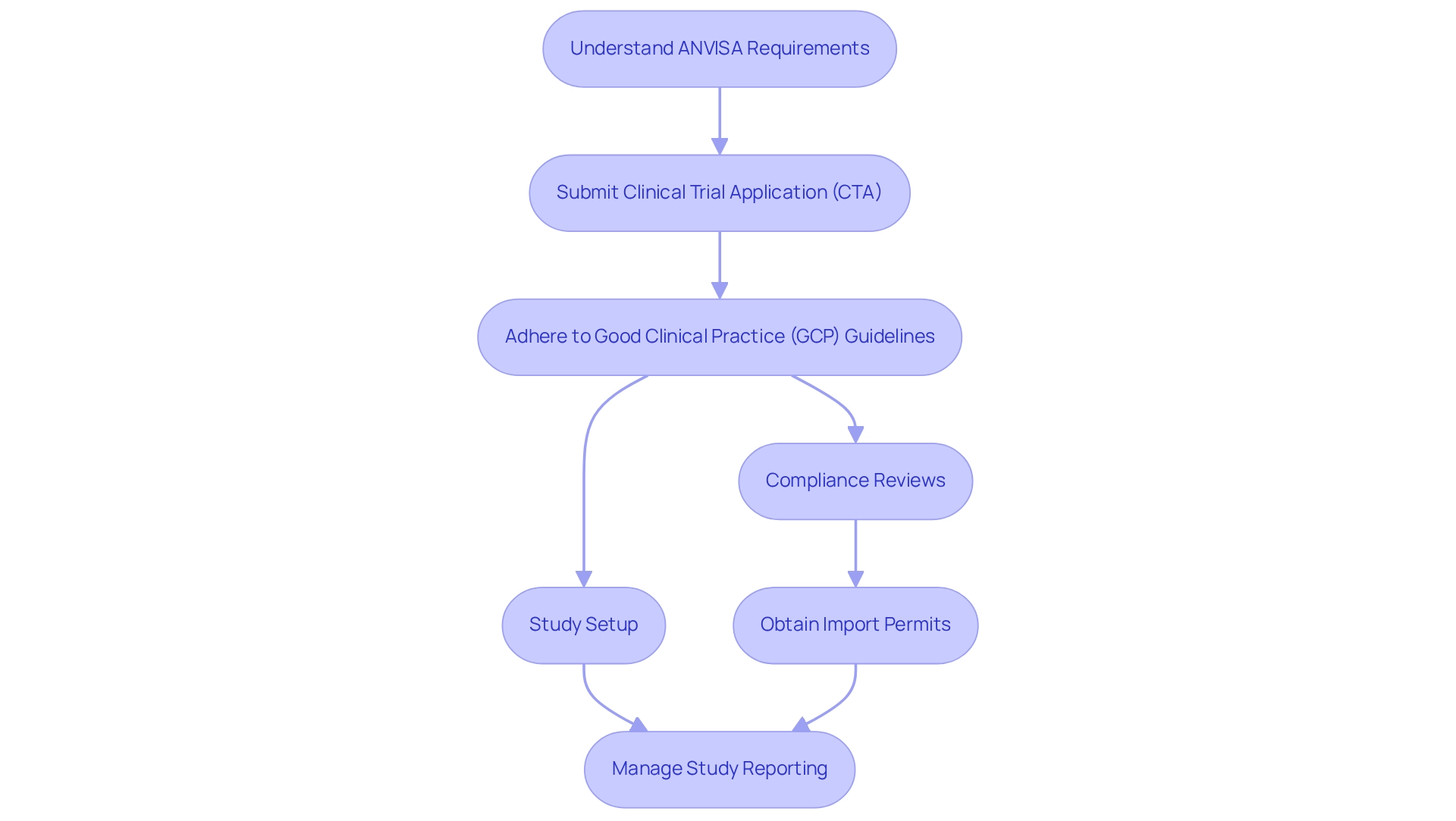
Brazil's oversight environment for health studies is primarily managed by the National Health Surveillance Agency (ANVISA) and various ethical committees, which play a crucial role in safeguarding the integrity and safety of medical research. Researchers must possess a thorough understanding of the specific requirements mandated by ANVISA, including the submission of a Clinical Trial Application (CTA) and strict adherence to Good Clinical Practice (GCP) guidelines. Mastery of these regulations is essential for navigating the approval process efficiently and effectively.
Recent legislative updates, particularly Law 14,874/2024, have significantly streamlined the framework, enhancing the speed of research approvals. This law introduces measures that facilitate quicker access to essential resources, such as automatic import licenses for accredited investigators, granted within five days following protocol approval. Staying informed about these advancements is vital for researchers to ensure compliance and avert potential delays in the initiation of studies.
Moreover, the regulatory landscape is evolving, with ANVISA's commitment to clarity and efficiency reflected in its research application statistics for 2025. As the agency continues to refine its processes, understanding the implications of these changes is critical for effective management. bioaccess® offers comprehensive clinical study management services, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Nationalization of investigational devices
- Project management
- Reporting (study status, inventory, serious and non-serious adverse events)
This ensures that researchers are equipped to navigate these complexities.
Case studies, such as the management of human biological material, underscore the significance of ethical considerations and participant consent in research, reinforcing the necessity for adherence to established guidelines. This law governs the storage and use of human biological material collected during research, ensuring ethical handling and participant consent, while also establishing guidelines for biobanks and biorepositories.
Expert opinions on Brazil's regulatory landscape highlight the importance of ongoing education and adaptation to new regulations. As President Luiz Inacio Lula da Silva stated, "a fixed deadline would violate participant rights and ethical principles of dignity, beneficence, and justice."
As the research environment in Brazil becomes increasingly dynamic, investigators must prioritize compliance strategies that align with ANVISA's requirements to facilitate the advancement of medical devices and ensure the ethical conduct of studies. With insights from experts such as Katherine Ruiz in compliance matters for medical devices and in vitro diagnostics, organizations can more effectively navigate these challenges.
Key Regulations Governing Clinical Trials in Brazil
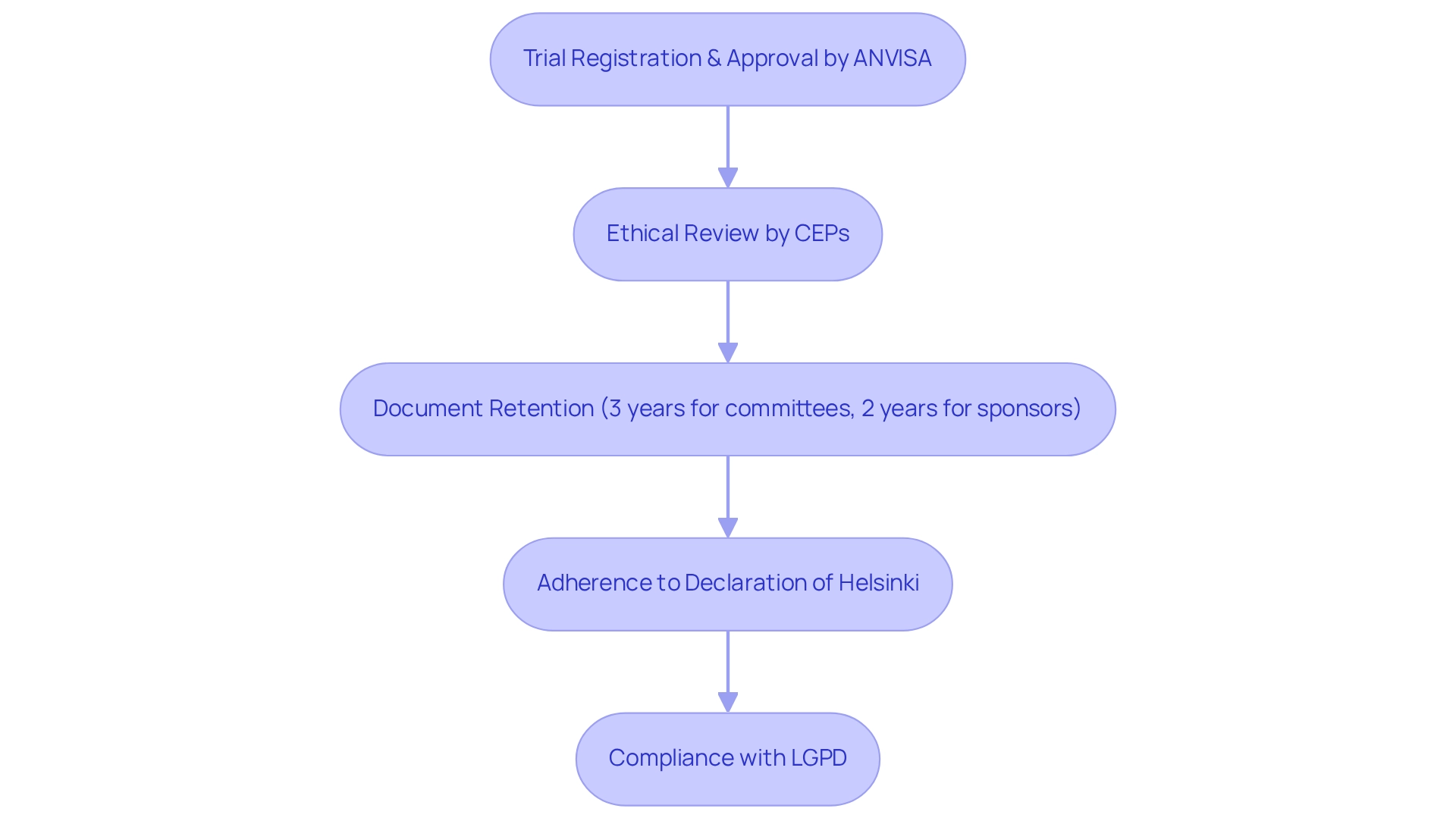
In Brazil, the regulatory environment for research studies is fundamentally shaped by compliance strategies specific to the country, as established by the Brazilian Health Regulatory Agency (ANVISA). These guidelines mandate that all medical trials must be registered and approved prior to initiation, thereby ensuring a structured and methodical approach to research. The ethical review process is conducted by local ethics committees, known as Comitês de Ética em Pesquisa (CEPs), which are tasked with assessing the ethical implications of proposed studies.
Importantly, these committees are required to retain all relevant records for three years following the conclusion of a study, underscoring the critical importance of transparency and accountability in clinical research. Additionally, sponsors must maintain essential documents for a minimum of two years after a marketing application is either approved or discontinued, which is vital for comprehending compliance requirements. Adherence to the World Medical Association's Declaration of Helsinki is also crucial, reinforcing the commitment to participant safety and ethical standards throughout the study process. Furthermore, researchers must navigate Brazil's General Data Protection Law (LGPD), which regulates the handling of personal data in research studies to ensure participant privacy is upheld.
bioaccess® offers extensive management services for studies, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project oversight
- Reporting
This comprehensive approach guarantees that every aspect of the research process is meticulously overseen, conforming to compliance standards and enhancing the overall effectiveness of execution.
Recent advancements, such as ANVISA's implementation of an improved analysis procedure for compliance submissions, highlight the agency's commitment to enhancing the efficiency of the oversight process. This new protocol allows for the use of documentation from comparable foreign oversight authorities (AREEs), facilitating quicker approvals for clinical trials while preserving rigorous safety and efficacy standards. As Diego Gomes, Senior Global Alliances Manager at Lhasa, noted, "The introduction of RDC 964/2025 aligns Anvisa’s guidelines with international standards for forced degradation studies, which necessitate a comprehensive scientific understanding of the drug product."
Moreover, ANVISA has recently instituted a new protocol for requests for authorization to import medicines and substances under special control, reflecting ongoing changes in the oversight landscape. As the regulatory environment evolves, awareness of Brazil-specific compliance strategies for trials becomes essential for successful research execution. bioaccess® is committed to maintaining information security and client trust, addressing any issues through our grievance and data protection protocols, thereby ensuring adherence and transparency throughout the research process.
Challenges in Clinical Trial Compliance in Brazil
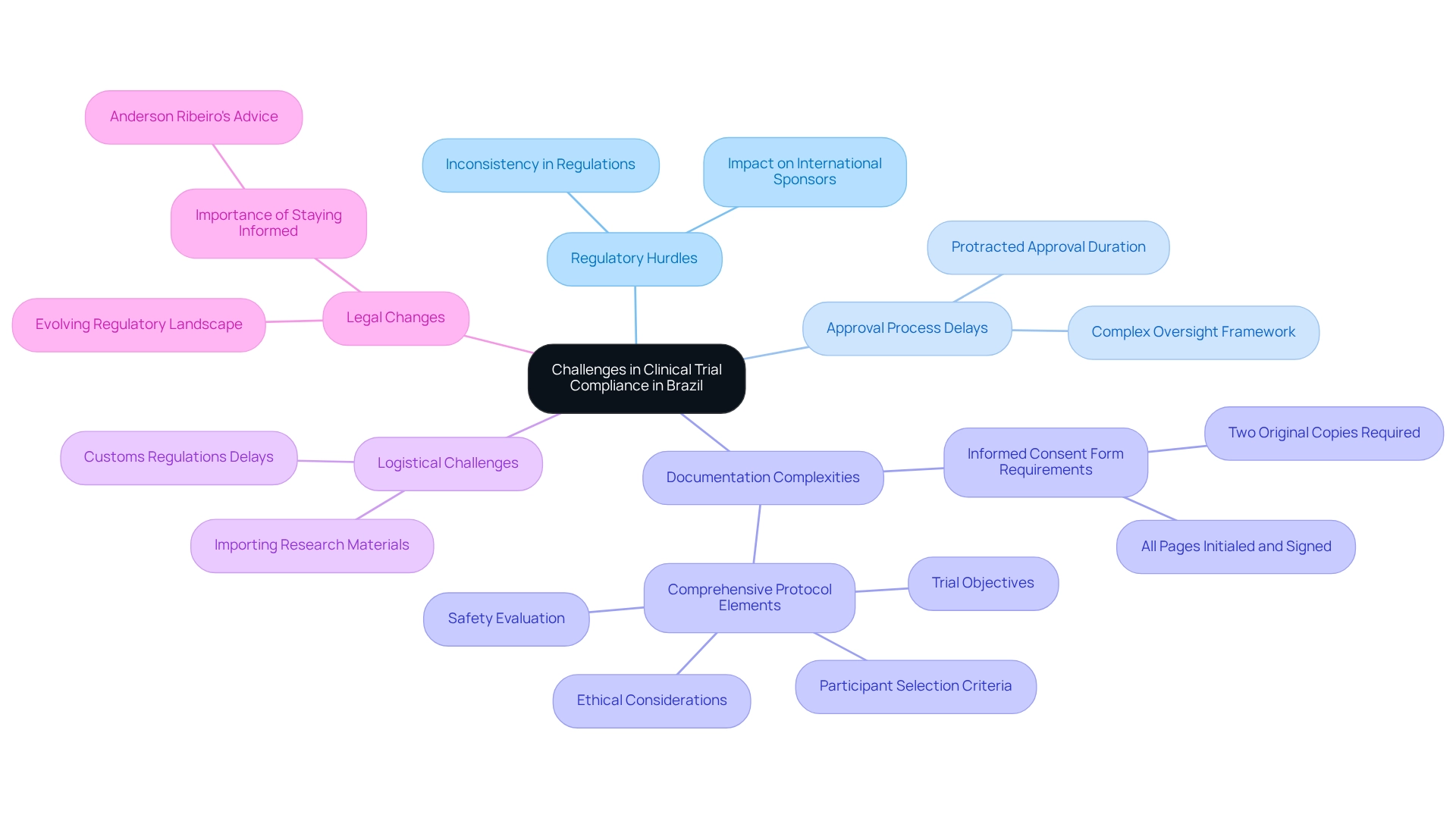
In Brazil, researchers frequently face significant regulatory hurdles that impede the progress of medical studies. A primary concern is the protracted approval process, which can substantially delay the initiation of studies. As of 2025, the average approval duration for medical studies in Brazil remains notably extended, often attributable to the intricate oversight framework and the varied interpretations of regulations by different ethics boards.
This inconsistency can result in confusion and delays, especially for international sponsors who may lack familiarity with local requirements. Additionally, the need for comprehensive documentation introduces another layer of complexity. For example, two original copies of the informed consent form (ICF) must be meticulously prepared, with all pages initialed and signed, which can prove to be a burdensome task. The evolving regulatory landscape, influenced by recent legislative changes, further complicates adherence efforts, necessitating that researchers remain vigilant about new developments.
As Anderson Ribeiro emphasizes, "Stay informed with the latest news on various areas of law and at Souto Correa," underscoring the critical importance of keeping abreast of legal changes. Furthermore, the research protocol must encompass essential elements such as study objectives, participant selection criteria, safety assessment, and ethical considerations, all of which are crucial for ensuring compliance and the integrity of the study.
Logistical challenges also play a pivotal role in compliance difficulties. Importing research materials can be fraught with challenges, as customs regulations may cause unexpected delays. These logistical obstacles can hinder the timely execution of trials, highlighting the necessity for meticulous planning and local expertise, such as that provided by bioaccess®, which boasts over 20 years of experience in Medtech.
A notable case study exemplifying these challenges is the technical note issued by ANVISA during the COVID-19 pandemic, which provided guidance for conducting research and bioequivalence studies affected by the crisis. This initiative supported researchers in navigating the extraordinary challenges posed by the pandemic, ensuring continuity in medical research despite the obstacles.
Understanding these intricate challenges is vital for developing effective Brazil-specific compliance strategies for trials that streamline the research process. By proactively addressing these issues, researchers can enhance their likelihood of successful execution, ultimately contributing to the advancement of medical technologies. This can be achieved through the comprehensive management services offered by bioaccess®, including feasibility studies, site selection, regulatory reviews, setup, import permits, project management, and reporting.
Best Practices for Ensuring Compliance in Clinical Trials
To ensure compliance in clinical trials in Brazil, researchers must adopt several best practices:
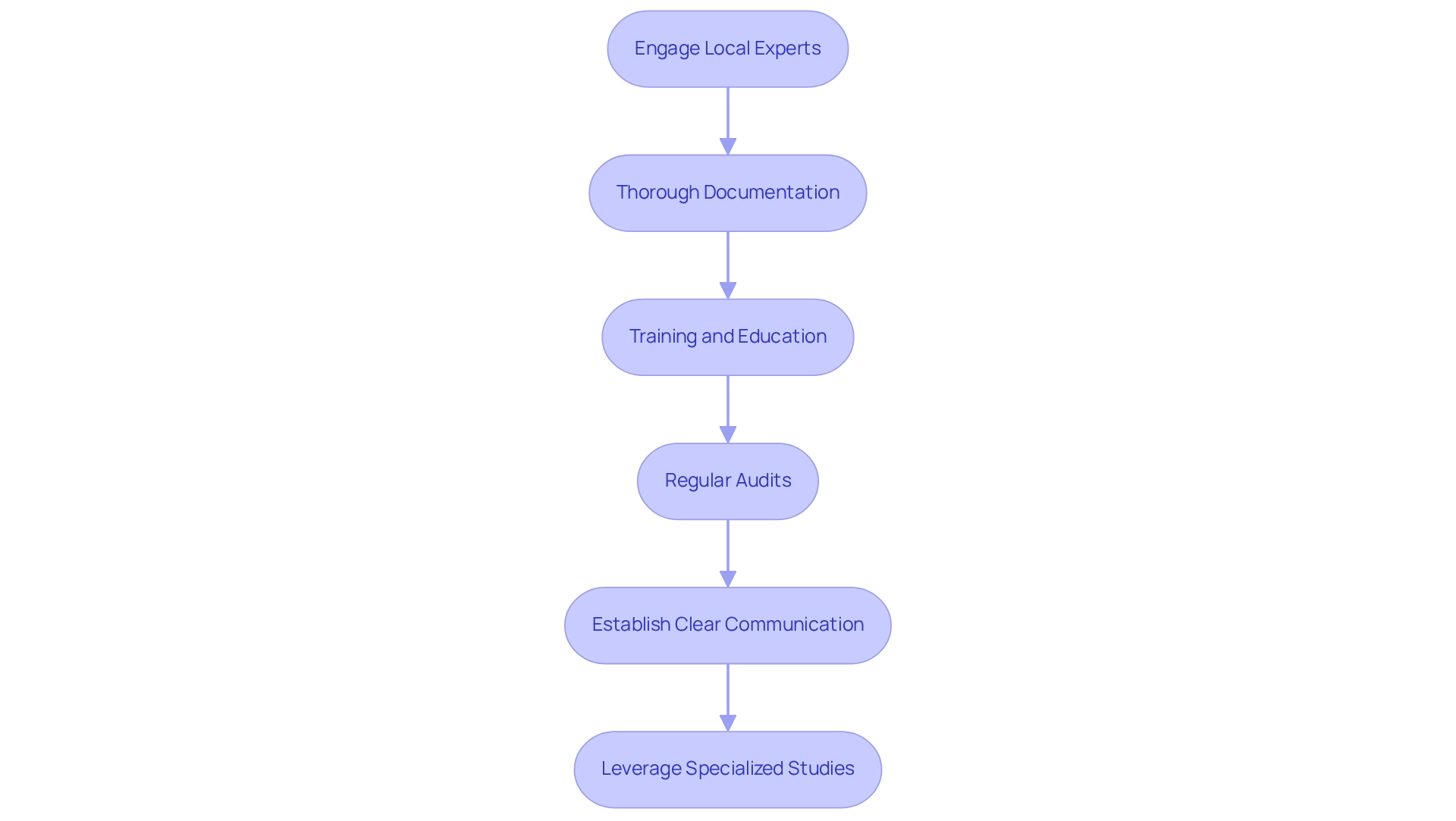
- Engage Local Experts: Collaborating with local compliance consultants is essential, as they possess a profound understanding of the intricate nuances of Brazilian regulations. Their expertise can significantly enhance the chances of success by effectively navigating the regulatory landscape, a service that bioaccess® excels in, given its extensive experience in the region.
- Thorough Documentation: Maintaining meticulous records of all communications and submissions to ethics committees and ANVISA is crucial. This practice guarantees adherence and promotes transparency and responsibility throughout the process, aligning with bioaccess®'s commitment to Brazil-specific compliance strategies for trials and comprehensive management services for studies.
- Training and Education: Providing comprehensive training for all team members on local regulations and Good Clinical Practice (GCP) guidelines is vital. This ensures that all participants are well-informed and able to follow the necessary standards, thus reducing risks of non-compliance. Bioaccess® highlights this aspect in its methodology for research studies, ensuring that teams possess the essential knowledge.
- Regular Audits: Conducting internal audits proactively helps identify potential compliance gaps. By addressing these issues early, researchers can mitigate risks and enhance the integrity of their clinical trials, which can be guided by Brazil-specific compliance strategies for trials supported by bioaccess®'s rigorous project management protocols.
- Establish Clear Communication: Fostering open lines of communication with regulatory bodies and ethics committees is essential for clarifying expectations and requirements. This proactive approach can lead to smoother interactions and quicker resolutions of any queries related to Brazil-specific compliance strategies for trials, a cornerstone of bioaccess®'s operational strategy.
- Leverage Specialized Studies: Bioaccess® specializes in managing various types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). Understanding these study types can assist researchers in coordinating their adherence strategies effectively.
In 2025, the significance of Brazil-specific compliance strategies for trials is underscored by the ongoing necessity for robust regulatory frameworks, particularly in light of recent challenges faced by clinical trials during the COVID-19 pandemic. For instance, ANVISA's release of a technical note during the pandemic sought to support ongoing research while addressing adherence challenges, emphasizing the necessity for flexibility and local knowledge in implementing Brazil-specific compliance strategies for trials. Furthermore, it is crucial to consider ethical implications, particularly for vulnerable populations, including those with incurable diseases and ethnic minorities, when developing compliance strategies.
As Negar Gharavi, Senior Director of Medical Writing & Compliance Affairs, stated, "BioPharma Services has successfully conducted several studies for the ANVISA submission," highlighting the importance of involving local compliance experts. Additionally, understanding the role of CNERS, which requires a quorum of nine members for voting on applications, provides further context on the regulatory landscape in Brazil. By implementing Brazil-specific compliance strategies for trials, researchers can enhance their compliance efforts and ultimately contribute to the successful advancement of medical technologies in Brazil.
For additional details on how bioaccess® can support your research requirements, please contact us.
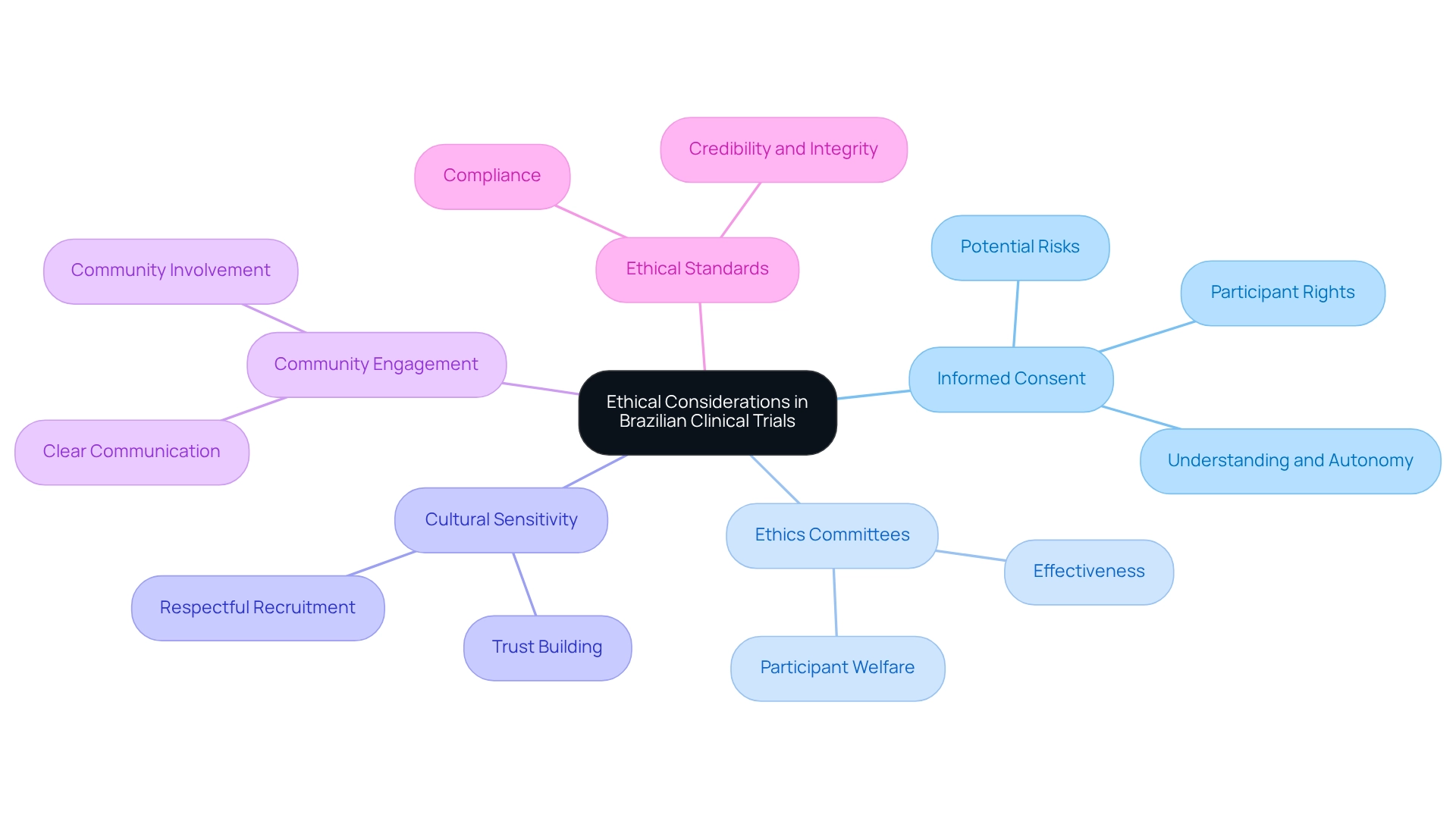
Ethical Considerations in Brazilian Clinical Trials
Ethical considerations in Brazilian clinical studies are paramount, shaped by the principles outlined in the WMA Declaration of Helsinki alongside local ethical guidelines. A primary focus must be on informed consent, necessitating that participants are thoroughly informed about the study's nature, potential risks, and their rights. In 2025, statistics reveal that adherence to informed consent protocols is under increasing scrutiny, with a notable rise in trials emphasizing participant understanding and autonomy. This trend reflects a growing acknowledgment of the significance of ethical practices in medical research.
The effectiveness of the ethical review process, conducted by local ethics committees, is crucial in ensuring participant welfare. These committees evaluate the ethical implications of research proposals, thereby safeguarding the rights and well-being of participants. Moreover, researchers must be attuned to the cultural context of their study populations, ensuring that recruitment practices are both respectful and inclusive. This cultural sensitivity not only fosters trust but also enhances participant engagement.
Informed consent practices in Brazil have evolved, with case studies underscoring the importance of clear communication and community involvement in the consent process. The challenges faced in diagnosing healthcare-associated infections (HAIs) in middle-income nations highlight the necessity for ethical considerations and community participation in research. Ethical considerations extend beyond mere compliance; they are integral to the credibility and integrity of the research.
As Ryan Jones, CEO & Co-Founder of Florence Healthcare, emphasizes, interoperable, site-centric technology can enhance relationships and accelerate timelines in research studies, further promoting ethical compliance. Additionally, Ai Ogata notes that improving education and collaboration in research is vital for supporting disaster-affected populations. This perspective underscores the importance of ethical instruction and education in medical studies in Brazil, reflecting the demand for more certified professionals as indicated by the Japanese Nursing Association.
By adhering to these ethical standards, researchers can contribute to the advancement of medical studies in Brazil while ensuring that participant rights are upheld and respected.
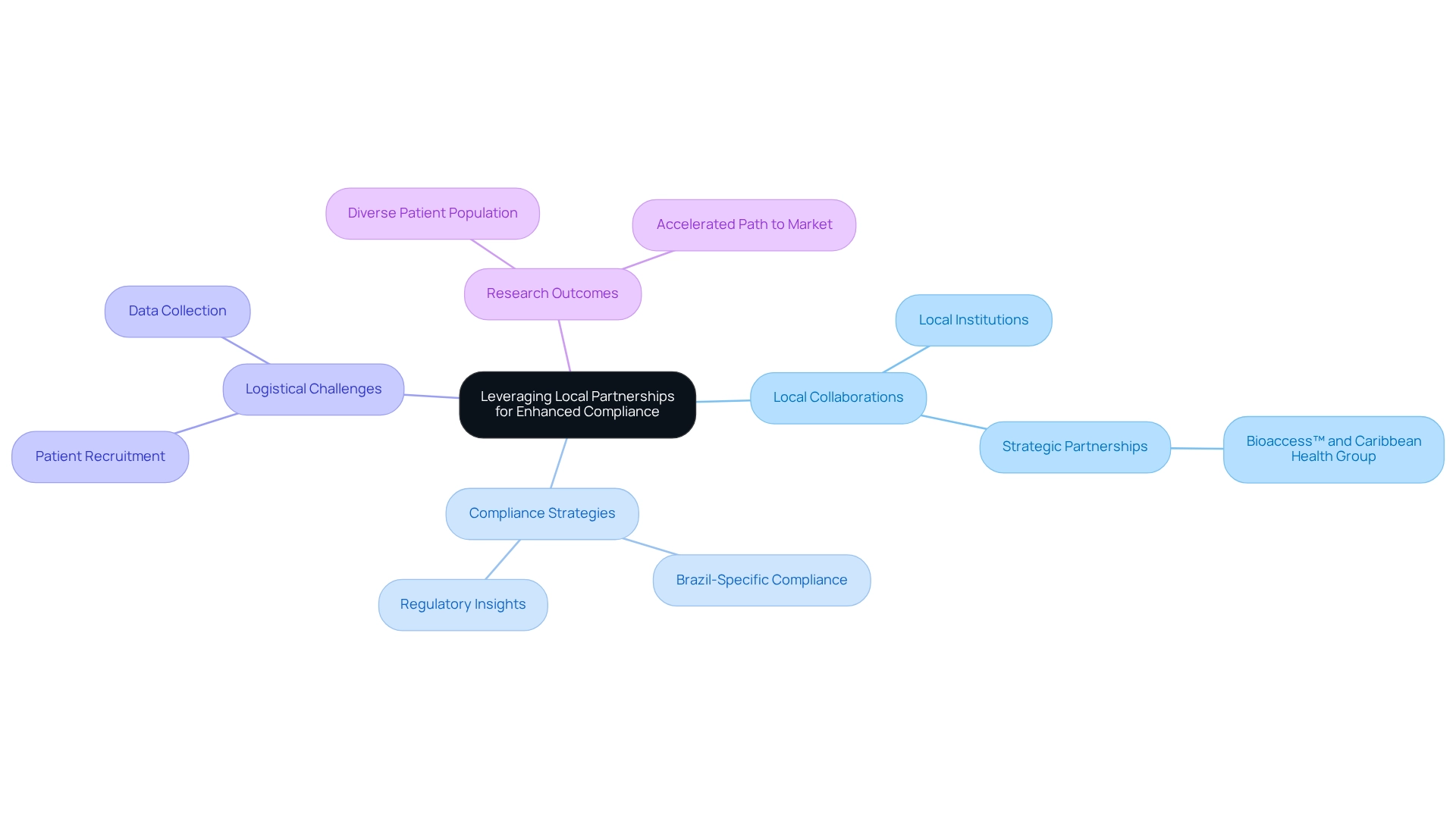
Leveraging Local Partnerships for Enhanced Compliance
Utilizing local collaborations is a crucial approach for enhancing adherence in Brazilian research studies through Brazil-specific compliance strategies for trials. Collaborating with local research institutions, hospitals, and universities is essential for developing these strategies, as it provides critical insights into the regulatory landscape and facilitates smoother interactions with ethics committees and regulatory bodies. In 2025, the emphasis on local collaborations is underscored by the growing acknowledgment of Brazil's diverse patient population, which is vital for comprehensive study outcomes, particularly in fields such as oncology where the worldwide disease burden is substantial.
Significantly, 52 percent of worldwide research studies occur beyond the U.S., emphasizing Brazil's importance as a research location. Local collaborators play an essential role in addressing logistical challenges, such as patient recruitment and data gathering, which frequently hinder study success. By building robust connections with local stakeholders, sponsors can foster trust and cooperation, resulting in more effective study procedures and improved adherence outcomes. The recent collaboration between bioaccess™ and Caribbean Health Group to establish Barranquilla as a prominent hub for medical studies in Latin America exemplifies how strategic partnerships can enhance research capabilities.
Backed by Colombia's Minister of Health, this initiative aims to simplify procedures and attract more research projects to the area. Expert opinions highlight that cooperation with local organizations not only streamlines adherence but also enhances research efficiency. Dr. Pankaj M. Madhani notes that a combination of resources, including supportive government policies and a skilled workforce, helps establish a sustainable advantage for research sectors. Researchers have found that engaging with local entities can lead to faster patient recruitment and more reliable data collection, ultimately accelerating the path to market for innovative medical technologies.
Moreover, the extensive services provided by bioaccess™, such as feasibility studies, site selection, regulatory reviews, setup for experiments, import permits, project management, and reporting, are crucial for managing the intricacies of research studies. As Brazil continues to position itself as a promising center for medical research, the benefits of local collaborations in research projects, particularly Brazil-specific compliance strategies for trials, become increasingly evident, leading to successful outcomes in the Medtech industry while contributing to job creation and economic development in local communities.
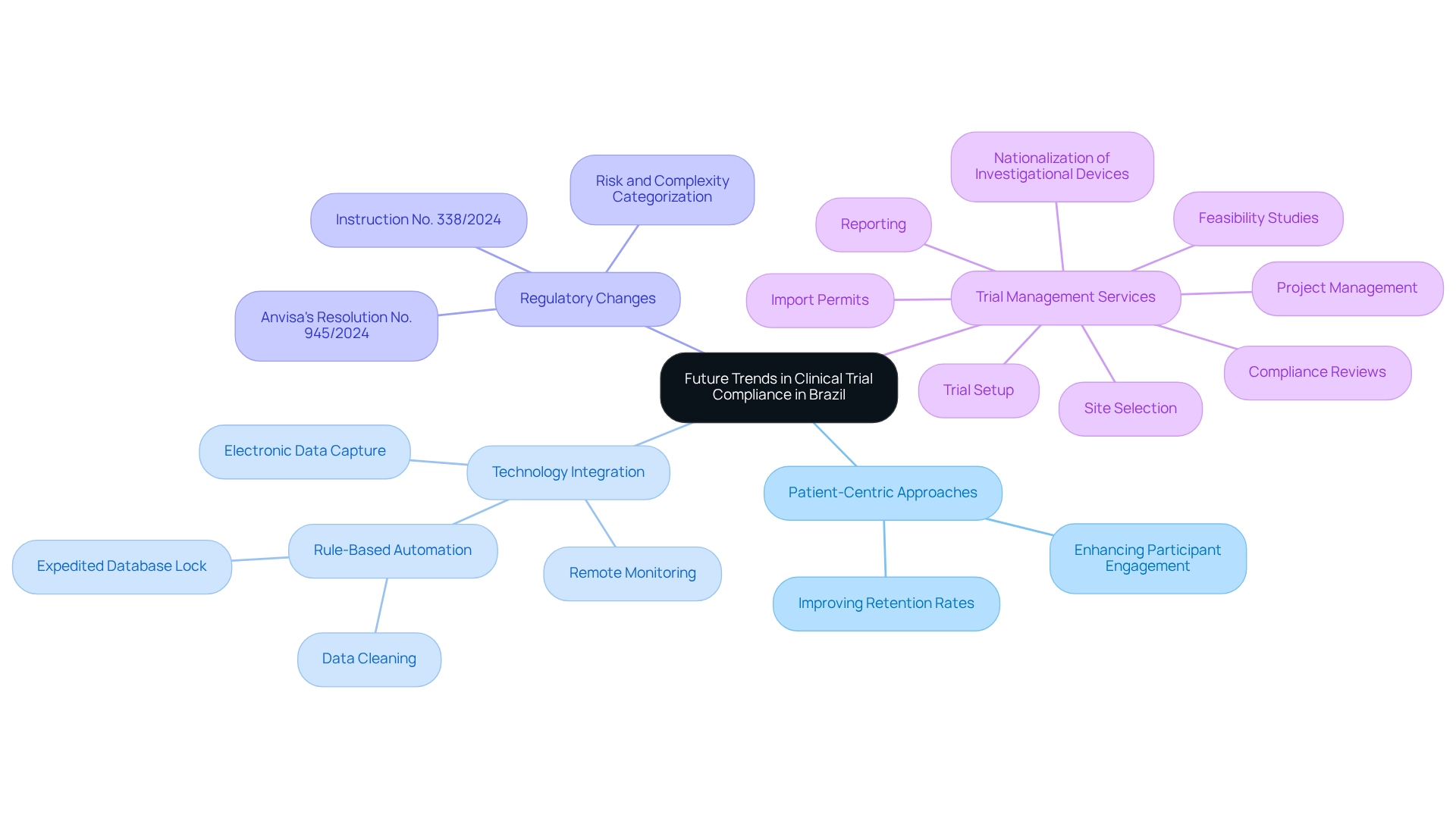
Future Trends in Clinical Trial Compliance in Brazil
The landscape of clinical study compliance in Brazil is on the brink of significant transformation, propelled by several key trends that pertain to Brazil-specific compliance strategies for trials. A burgeoning emphasis on patient-centric approaches is likely to cultivate more adaptable regulatory frameworks that prioritize participant engagement and retention. This shift not only enhances the participant experience but also improves overall study outcomes.
For instance, the integration of technology, such as electronic data capture and remote monitoring, is set to simplify regulatory processes and bolster data integrity, rendering trials more efficient and trustworthy. Notably, GSK's implementation of rule-based automation for data cleaning has expedited the time to database lock, exemplifying how technology can enhance compliance efficiency.
Moreover, as the regulatory environment evolves, researchers must remain vigilant regarding ongoing changes, particularly those related to Brazil-specific compliance strategies for trials and global standards. The recent publication of Anvisa's Resolution No. 945/2024 and Instruction No. 338/2024 underscores the importance of adapting to new regulations that categorize risk and complexity in research studies. These advancements reflect a commitment to enhancing the research framework in Brazil, ensuring alignment with Brazil-specific compliance strategies for trials while meeting both local and global standards.
Incorporating patient-centric strategies not only aligns with regulatory trends but also mirrors a broader industry movement toward prioritizing the needs and experiences of participants. A case study titled "Enhancing Site Experience through Technology" illustrates how the integration of technology streamlines the site experience, alleviating the burden on research associates (CRAs) and improving data entry processes, ultimately benefiting patient care and study outcomes.
As we approach 2025, it is crucial for researchers to stay informed about these emerging trends, as they will be pivotal for upholding regulations and effectively navigating the evolving research landscape in Brazil, particularly concerning Brazil-specific compliance strategies for trials. Furthermore, bioaccess offers extensive trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Nationalization of investigational devices
- Project management
- Reporting
These services are essential for advancing global health through international collaboration and innovation in medtech. Insights from industry leaders, such as Ibrahim Kamstrup-Akkaoui, who highlighted the potential of AI initiatives to generate meaningful test data, emphasize the innovative strategies being adopted in the industry, further underscoring the impact of medtech clinical studies on local economies through job creation, economic growth, and healthcare enhancement.
Conclusion
As Brazil's clinical trial landscape continues to evolve, a deep understanding of its regulatory framework is essential for researchers seeking to conduct successful studies. The oversight provided by the National Health Surveillance Agency (ANVISA) ensures that adherence to established guidelines is prioritized. Recent legislative changes, including Law 14,874/2024, have further streamlined processes to facilitate quicker approvals. Nevertheless, the complexities of navigating ethical considerations and upholding rigorous documentation standards remain significant challenges for compliance.
Engaging local expertise is paramount. Collaboration with local institutions and regulatory consultants can greatly enhance the understanding of Brazilian regulations, leading to more efficient trial execution. Implementing best practices—such as thorough documentation, regular audits, and clear communication with ethics committees—will fortify compliance efforts. Ethical considerations, particularly concerning informed consent and participant welfare, must always be at the forefront of clinical research to uphold the integrity of studies and safeguard participant rights.
Looking ahead, the future of clinical trial compliance in Brazil is poised to be influenced by a stronger focus on patient-centric approaches and the integration of technology. As researchers adapt to these emerging trends, it is crucial that they remain vigilant and proactive in their compliance strategies. By leveraging comprehensive clinical trial management services, researchers can adeptly navigate the complexities of the regulatory environment, ultimately contributing to advancements in medical technologies and improved healthcare outcomes in Brazil. A commitment to ongoing education and ethical practices will be vital in ensuring that Brazil continues to thrive as a key player in the global clinical trial arena.
Frequently Asked Questions
What organization primarily manages the oversight environment for health studies in Brazil?
The oversight environment for health studies in Brazil is primarily managed by the National Health Surveillance Agency (ANVISA) and various ethical committees.
What is required from researchers before conducting medical research in Brazil?
Researchers must submit a Clinical Trial Application (CTA) and adhere to Good Clinical Practice (GCP) guidelines, along with a thorough understanding of ANVISA's specific requirements.
How has recent legislation, specifically Law 14,874/2024, impacted research approvals in Brazil?
Law 14,874/2024 has streamlined the approval process, allowing for quicker access to essential resources, such as automatic import licenses granted within five days after protocol approval.
What services does bioaccess® provide to assist researchers in Brazil?
Bioaccess® offers comprehensive clinical study management services, including feasibility assessments, site selection, compliance reviews, study setup, import permits, nationalization of investigational devices, project management, and reporting.
What ethical considerations are important in research involving human biological material in Brazil?
Ethical considerations include ensuring participant consent and adherence to established guidelines for the storage and use of human biological material, as well as guidelines for biobanks and biorepositories.
What is the role of local ethics committees in Brazil's research environment?
Local ethics committees, known as Comitês de Ética em Pesquisa (CEPs), assess the ethical implications of proposed studies and are required to retain all relevant records for three years after a study concludes.
What international ethical standard must researchers in Brazil adhere to?
Researchers in Brazil must adhere to the World Medical Association's Declaration of Helsinki, which emphasizes participant safety and ethical standards throughout the study process.
What law regulates the handling of personal data in research studies in Brazil?
Brazil's General Data Protection Law (LGPD) regulates the handling of personal data in research studies to ensure participant privacy is upheld.
How does ANVISA facilitate quicker approvals for clinical trials?
ANVISA has implemented improved analysis procedures for compliance submissions and allows the use of documentation from comparable foreign oversight authorities, which facilitates quicker approvals while maintaining safety and efficacy standards.
What is the significance of ongoing education and adaptation to new regulations in Brazil's research landscape?
Ongoing education and adaptation are crucial for researchers to effectively navigate compliance strategies that align with ANVISA's requirements and to ensure the ethical conduct of studies.




