Overview
Collaboration in medtech trial design is essential for fostering innovation and enhancing patient outcomes. By integrating diverse perspectives from stakeholders—such as researchers, regulatory authorities, and healthcare professionals—this collaborative approach addresses critical challenges in clinical research. Effective teamwork not only accelerates the development of medical devices but also improves data quality and participant engagement. Successful partnerships exemplify how collaboration leads to quicker market entry and higher compliance rates, underscoring the necessity of a united front in advancing healthcare solutions.
Introduction
In the rapidly evolving landscape of medical technology, the success of clinical trials hinges on one crucial element: collaboration. By uniting diverse stakeholders—including researchers, regulatory bodies, and healthcare professionals—effective partnerships pave the way for innovative trial designs that enhance patient outcomes. This article delves into the significance of collaboration in Medtech trial design, exploring proven strategies, key stakeholder roles, and the impact of technology on fostering teamwork. As the industry navigates complex regulatory environments and strives for efficiency, understanding the dynamics of collaboration becomes essential for advancing medical devices and driving meaningful improvements in healthcare delivery.
The Importance of Collaboration in Medtech Trial Design
Collaboration in medtech trial design stands as a fundamental pillar in the creation of successful Medtech studies, uniting a diverse array of stakeholders—including researchers, regulatory authorities, and healthcare professionals—all striving towards a shared objective. This collaborative method underscores the significance of teamwork in medtech trial design, fostering innovation and enhancing communication while ensuring that a multitude of perspectives is integrated into the design process. The synergy generated through effective teamwork can significantly expedite the development of medical devices while simultaneously enhancing the quality of data collected.
This, in turn, leads to superior patient outcomes.
For instance, bioaccess™ and Caribbean Health Group (CHG) have joined forces to establish Barranquilla as a prominent location for medical studies in Latin America, a decision supported by Colombia's Minister of Health. This partnership aims to attract additional research initiatives to the region, thereby improving the overall environment for Medtech studies. Furthermore, GlobalCare Clinical Studies has collaborated with bioaccess™ to enhance ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and impressive 95% retention rates.
Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, shared his positive experience with bioaccess® during its inaugural human trial in Colombia, emphasizing the effectiveness of their collaborative approach. Additionally, Dr. John B. Simpson's research on Avinger's OCT-guided atherectomy in Cali, Colombia, exemplifies the fruitful partnerships with LATAM CRO experts that propel innovation in clinical research.
Expert opinions indicate that collaboration in medtech trial design can help mitigate common challenges faced in Medtech studies, such as regulatory complexities and patient recruitment difficulties. As Elizabeth V. Eikey observed, "To assess how HIT influences teamwork and how we can develop HIT to effectively support teamwork, we require additional studies that specifically address cooperative issues."
The impact of teamwork on research outcomes is substantial. Trials characterized by robust collaboration in medtech trial design not only achieve higher enrollment rates but also demonstrate improved retention and compliance among participants. By leveraging the collective knowledge of all parties involved—including bioaccess®'s extensive experience in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies—medical technology companies can navigate the intricate landscape of research more effectively, ultimately advancing medical devices to market more swiftly and successfully.
Key Stakeholders in Medtech Trials and Their Roles
In Medtech studies, collaboration in trial design among key stakeholders—clinical researchers, regulatory agencies, sponsors, and patients—is essential for success. Each group contributes uniquely to the study process: clinical researchers are responsible for designing and conducting the studies, ensuring robust methodologies that comply with industry standards. Regulatory agencies oversee compliance with safety regulations, a vital component for maintaining public trust and ensuring patient safety.
Notably, the European Medical Device Regulation, effective since 2020, has introduced significant changes affecting how studies are conducted and regulated. Sponsors, often pharmaceutical corporations or Medtech companies, provide essential funding and resources to enable these studies, while patients offer invaluable insights into the usability and effectiveness of the medical devices being assessed.
The importance of patient involvement cannot be overstated. Involving patients early in the study design process can lead to more patient-centered outcomes, ultimately enhancing recruitment rates and retention throughout the research. Statistics suggest that studies with active patient participation experience a notable rise in participant engagement, which is crucial for the success of any research.
However, small enterprises frequently encounter difficulties in managing the expenses related to sharing research data, which can obstruct partnership and openness.
Establishing clear communication channels and defining the roles of each stakeholder are fundamental strategies for fostering collaboration in Medtech trial design. For example, bioaccess provides extensive management services for studies, encompassing feasibility assessments, site selection, compliance evaluations, setup, import permits, nationalization of investigational devices, project management, and reporting. Recent initiatives by industry associations have highlighted the importance of transparency in medical research data sharing, building trust among stakeholders and enhancing the overall quality of research.
A significant example is the Bill & Melinda Gates Foundation's dedication to enhancing data accessibility in resource-limited nations, underscoring the global trend towards responsible data utilization in research. Additionally, a Japanese respondent expressed a common concern: "Will the results of the clinical study be provided?" This reflects a critical need for transparency and patient involvement in the process.
As the landscape of medical technology studies evolves, understanding the interactions between these participants will be crucial. By promoting a cooperative atmosphere and emphasizing patient participation, collaboration in Medtech trial design enables medical technology firms to navigate the intricacies of clinical studies more efficiently, ultimately leading to the successful progression of pioneering medical devices.
Proven Strategies for Effective Collaboration in Medtech Trials
To promote effective teamwork in Medtech studies, stakeholders can adopt various established strategies that significantly enhance results through collaboration in medtech trial design. First, establishing regular communication through scheduled meetings and updates is crucial to ensure all parties remain aligned on goals and progress. This approach not only facilitates transparency but also builds trust among team members.
Second, utilizing collaborative tools and platforms that enable real-time data sharing and feedback can streamline processes and improve efficiency. These tools provide immediate access to critical information, which is essential for timely decision-making.
Third, creating a culture of inclusivity is vital. When all stakeholders feel valued and heard, it encourages open dialogue and the exchange of innovative ideas. This inclusive environment can lead to more creative solutions and a stronger team dynamic.
Fourth, defining clear roles and responsibilities helps avoid overlaps and ensures accountability within the team. This clarity is essential for maintaining focus and efficiency throughout the process.
Fifth, participating in joint training sessions can build rapport and understanding among team members, improving teamwork. Surveys indicate a growing demand for flexible, accessible training relevant to clinical study management, underscoring the importance of continuous learning in this field.
Moreover, studies indicate that collaboration in medtech trial design can enhance patient outcomes by pooling resources and expertise, further emphasizing the benefits of teamwork in Medtech research. Significantly, studies utilizing a dedicated study manager had an odds ratio of 3.80 for successful recruitment, underscoring the impact of structured management on study success. Furthermore, bioaccess® provides extensive management services for research studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and reporting, all essential for successful outcomes.
Our expertise also encompasses review and feedback on study documents to comply with country requirements, ensuring that all regulatory aspects are met.
Additionally, a case study titled "Business Management Perspective in Clinical Research" suggests that applying business management principles can enhance recruitment and overall study success, reinforcing the necessity for a structured approach. By adopting these best practices, healthcare companies can substantially enhance their research designs and results, focusing on collaboration in medtech trial design to advance global health improvement through international cooperation and innovation in medical technology. As noted by Haleema Shakur, "Barbara Farrell, Sara Kenyon and Haleema Shakur contributed equally to this work," reflecting the collaborative spirit essential for success in clinical research.
Overcoming Challenges in Medtech Trial Collaborations
Collaboration in medtech trial design presents significant challenges, including miscommunication, conflicting stakeholder priorities, and complex regulatory requirements imposed by organizations such as the FDA and EMA. To effectively navigate these obstacles, it is crucial for all parties involved to prioritize transparency and establish a unified vision from the outset. Regular check-ins and updates are essential in identifying potential issues early, allowing for timely interventions before they escalate into larger conflicts.
Creating a structured conflict resolution framework is another vital strategy. This framework should promote open dialogue and constructive discussions, enabling stakeholders to address disagreements in a manner that fosters collaboration rather than division. A systematic review emphasized the necessity of training to enhance the quality of randomized research studies, underscoring the importance of equipping teams with the skills needed for effective communication.
Statistics indicate that miscommunication is a prevalent issue in research studies, leading to delays and inefficiencies. By utilizing tools that facilitate seamless integration of health records, such as bioaccess®’s Rave Companion and Medidata Link, stakeholders can enhance decision-making and simplify study arrangements. These tools not only improve communication but also alleviate administrative burdens on healthcare providers, ultimately resulting in better patient outcomes.
The integration of health and medical data is crucial for creating cohesive healthcare experiences, as demonstrated in case studies where effective health record integration led to improved clinical outcomes. Furthermore, expert perspectives reveal that overcoming obstacles in Medtech study partnerships requires a collaborative approach in trial design and a proactive strategy. Ed Ball, Manager of Intelligence & Strategic Execution, emphasizes the importance of addressing equity challenges in MedTech, which can complicate collaboration efforts. Case studies illustrate that organizations fostering a culture of adaptability and problem-solving are better positioned to achieve their goals.
At bioaccess®, we are committed to ensuring information security and client trust through our comprehensive grievance and data protection procedures. For any queries or concerns regarding the processing of your information, please contact our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), located at 1200 Brickell Avenue, Suite 1950 #1034, or via email at info@bioaccessla.com. Our expertise in managing various studies—including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF)—positions us to support stakeholders effectively.
By adopting these optimal methods, stakeholders can improve teamwork, reduce disputes, and achieve positive results in medical technology studies.
Leveraging Technology to Enhance Collaboration in Medtech Trials
Collaboration in medtech trial design is essential for fostering teamwork in Medtech studies, significantly enhancing the efficiency and effectiveness of medical research. Electronic data capture (EDC) systems, project management software, and virtual communication platforms are leading this transformation, enabling seamless information sharing and real-time engagement among all stakeholders involved. A centralized data management system, for example, allows multiple parties to access and update research data simultaneously, thereby minimizing the risk of errors and miscommunication that can disrupt a study.
bioaccess® specializes in comprehensive research management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
This expertise is vital in navigating the complexities of medical studies, particularly in Latin America, where the company has a proven track record in managing:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Medical Follow-Up Studies (PMCF)
Despite these advancements, challenges persist in Medtech studies, such as the digital divide impacting recruitment and concerns surrounding data privacy and integrity. The integration of virtual meeting tools has revolutionized team communication, facilitating regular check-ins and discussions free from geographical constraints. This is particularly significant in the context of the ongoing digital transformation in healthcare, where ethical considerations regarding participant consent and data privacy are of utmost importance.
A recent study on ethical considerations in digital health assessments highlights the necessity of establishing clear ethical guidelines and data standards to ensure participant trust and data reliability.
The financial aspects of health assessments also play a critical role, with 510(k) application fees typically falling below $10,000, while PMA fees exceed two hundred thousand dollars. The impact of electronic data collection on research efficiency is substantial. By optimizing data collection and management processes, EDC systems support quicker decision-making and enhance collaboration in medtech trial design among research teams.
As we approach 2025, the latest tools for research management are expected to further enhance these capabilities, enabling more agile and responsive study designs. Mark Carol, MD, emphasizes that recognizing unknown factors sooner rather than later is crucial when aiming to reduce the time and cost of introducing a new venture. In this evolving landscape, collaboration in medtech trial design is vital for healthcare technology firms to understand the implications of technology on teamwork, ultimately expediting their clinical studies and bringing innovative medical devices to market more efficiently.
Current applications of AI in healthcare, such as experiments for antibiotic dosing and diagnostic decision-making, further illustrate the potential of technology to enhance medical technology studies. Moreover, the successful implementation of these tests by bioaccess® not only fosters healthcare innovation but also bolsters local economies through job creation and global partnerships.
Navigating Regulatory Considerations in Collaborative Medtech Trials
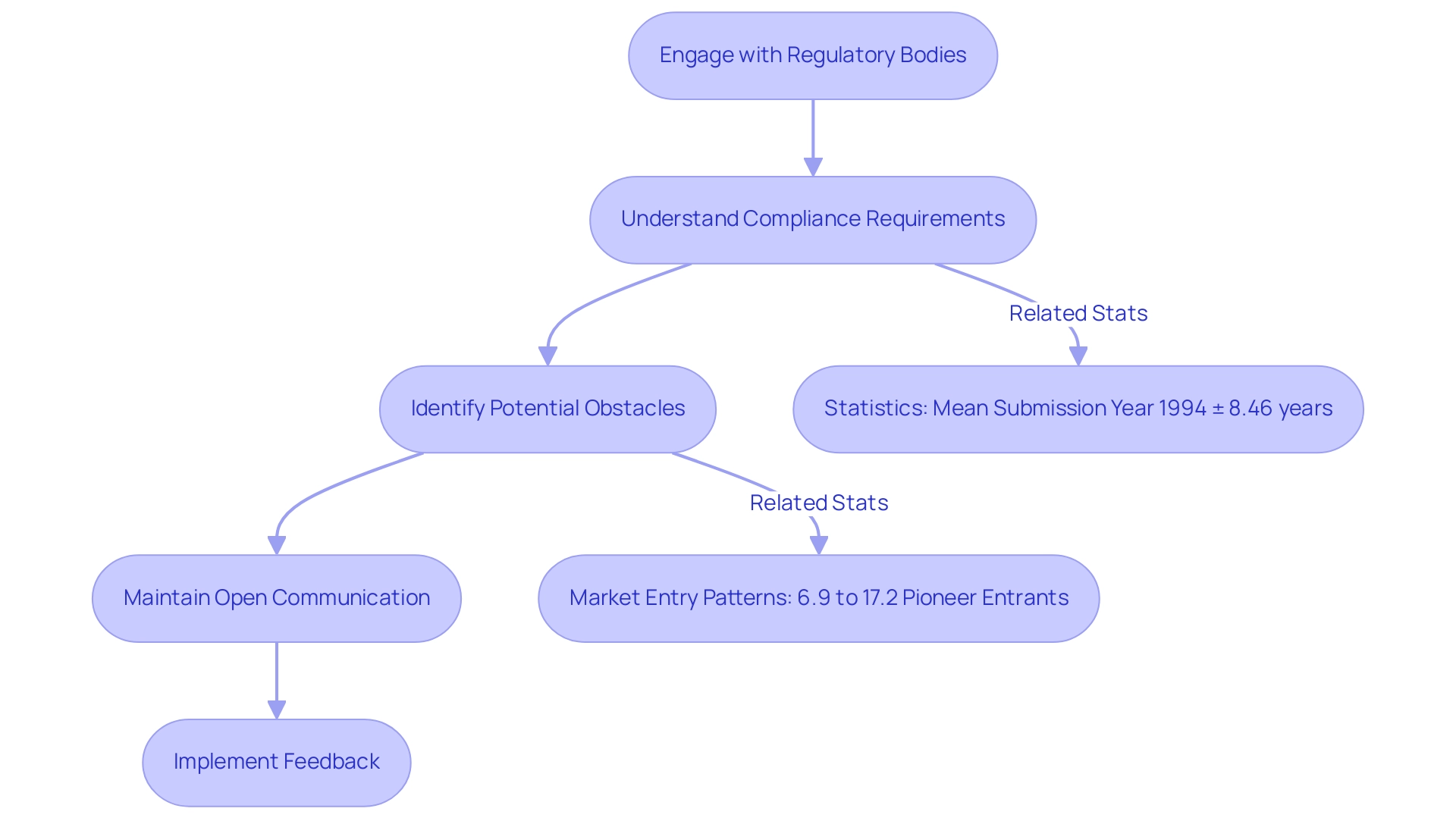
Navigating regulatory considerations is paramount in the collaboration on medtech trial design. Engaging with regulatory bodies at the outset of the design process is essential for understanding compliance requirements and expectations. This proactive strategy not only aids in identifying potential obstacles but also fosters smoother interactions with regulators.
For instance, the situation with Acorn Cardiovascular illustrates how regulatory uncertainty can lead to significant delays; the company faced a three-year setback and incurred additional expenses due to FDA recommendations for larger clinical studies. Moreover, maintaining open lines of communication with regulatory agencies throughout the process is crucial. This ensures that any modifications or updates are addressed promptly, thereby enhancing the credibility and success of the process.
Statistics reveal that the mean submission year for medical devices is 1994, with a standard deviation of 8.46 years, underscoring the lengthy timelines and variability often associated with the approval process. Furthermore, early engagement with regulatory bodies can significantly impact study outcomes. A study on market entry patterns among small firms in the medical device sector found that only 6.9% to 17.2% of these firms entered new device markets as pioneers, primarily due to regulatory uncertainties.
In contrast, firms that engaged early with regulators were more likely to navigate these challenges effectively. Notably, the rate of non-approvals in the PMA process is minimal, indicating that proactive regulatory engagement can lead to successful outcomes. By prioritizing regulatory compliance in collaboration on medtech trial design, stakeholders can enhance the credibility of their studies and improve their chances of successful market entry.
At bioaccess, our comprehensive research management services encompass:
- Feasibility studies
- Site selection
- Compliance assessments
- Study setup
- Import permits
- Project management
- Reporting
We also provide review and feedback on study documents to ensure compliance with country requirements and reporting on serious and non-serious adverse events. With experts like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, we ensure our clients are well-equipped to navigate the complexities of regulatory landscapes.
As noted by The Lancet, integrating the data, experience, and lessons learned from regulatory bodies can further support this endeavor.
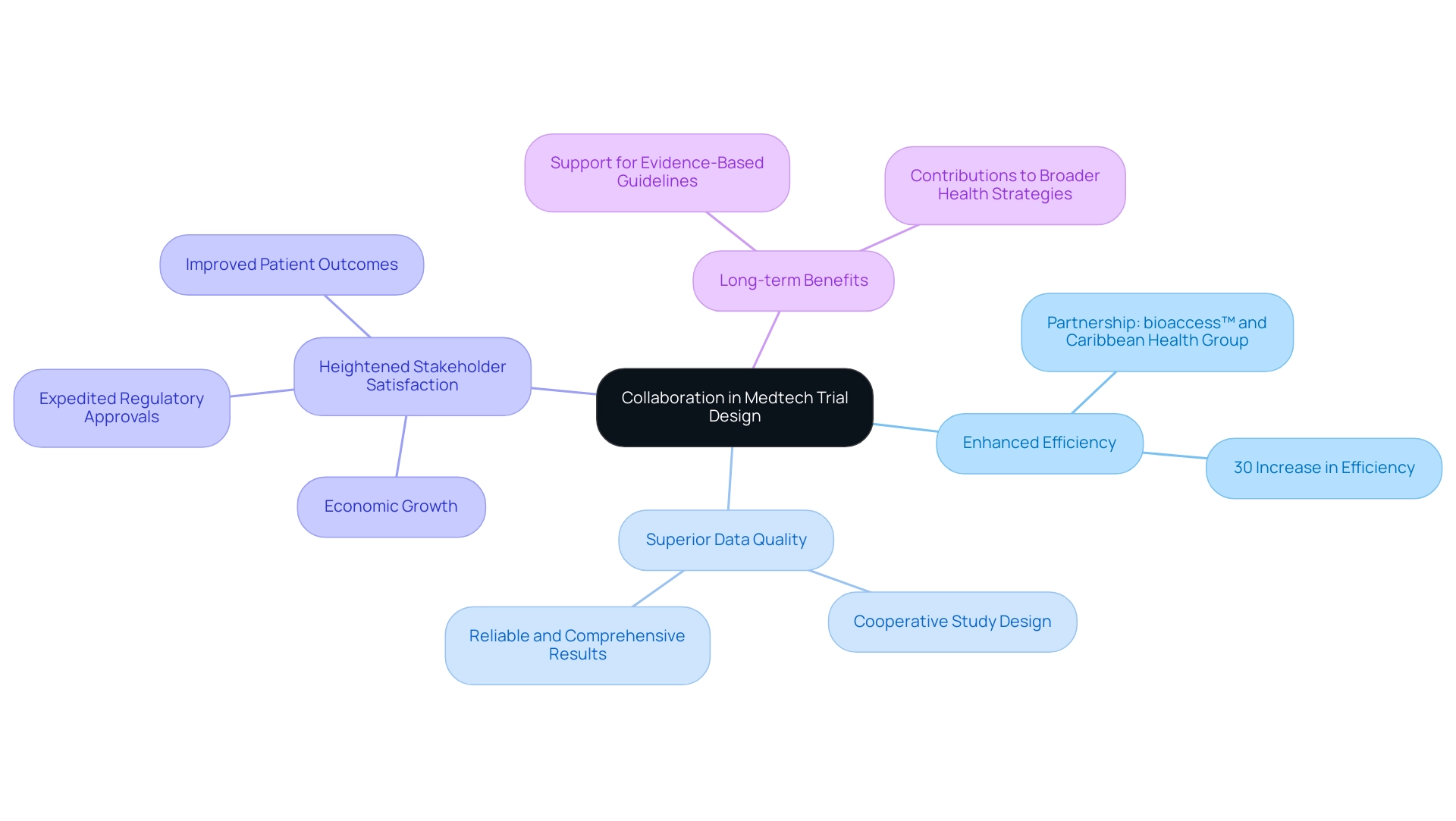
The Benefits of Successful Collaboration in Medtech Trial Design
Collaboration in medtech trial design is pivotal, yielding significant benefits such as enhanced efficiency, superior data quality, and heightened stakeholder satisfaction. By uniting diverse expertise from various stakeholders, challenges can be addressed more effectively, fostering innovative solutions that ultimately improve patient outcomes. A notable instance is the partnership between bioaccess™ and Caribbean Health Group, revealed on March 29, 2019, which seeks to establish Barranquilla as a prominent location for clinical studies in Latin America, backed by Colombia's Minister of Health.
This initiative not only enhances the local research landscape but also contributes to economic growth and healthcare improvement in the region. Collaborative studies are often associated with expedited regulatory approvals and quicker market entry, as they present a thorough approach to demonstrating safety and efficacy. For instance, historical partnerships in England aimed at reducing health inequalities illustrate how alliances can contribute to broader health strategies, even if direct health outcome improvements are not immediately evident. These partnerships emphasize the complexities involved and the potential for indirect advantages over time.
Statistics indicate that successful partnerships can result in a 30% increase in efficiency, significantly shortening timelines and expenses. Moreover, expert opinions emphasize that collaboration improves data quality, with studies suggesting that experiments designed through cooperative efforts produce more reliable and comprehensive results. As Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, remarked about his experience with bioaccess® during its first human study in Colombia, the incorporation of local expertise and resources is vital for success.
This highlights the significance of teamwork in attaining improved results. Additionally, successful research studies result in new medications and treatments that enhance patient outcomes and support evidence-based guidelines and policies in healthcare. bioaccess™ provides extensive service capabilities, including study setup, project management, and compliance reviews, which are vital for the success of clinical studies.
As the Medtech landscape evolves in 2025, collaboration in medtech trial design will continue to shape the industry, driving advancements that benefit not only individual trials but also the sector as a whole. Collaboration in medtech trial design is essential for prioritizing collaborative approaches that foster innovation and ensure that medical devices reach the market more swiftly, ultimately enhancing healthcare delivery.
Conclusion
The exploration of collaboration in Medtech trial design underscores its critical role in enhancing the efficacy and success of clinical studies. By uniting key stakeholders—researchers, regulatory bodies, sponsors, and patients—collaborative efforts create a dynamic environment where innovative solutions can thrive. Statistics highlight the tangible benefits of such partnerships, including increased trial efficiency and improved data quality, both vital for advancing medical devices and enhancing patient outcomes.
Moreover, the integration of technology has revolutionized stakeholder communication and data sharing, streamlining processes and enabling real-time collaboration. As the industry evolves, establishing clear communication channels and defining roles becomes paramount. By prioritizing transparency and inclusivity, Medtech companies can navigate the complexities of clinical trials more effectively.
Ultimately, successful collaboration not only expedites regulatory approvals but also enhances healthcare delivery as a whole. Various case studies illustrate that the collective expertise and resources pooled through collaboration can lead to groundbreaking advancements in medical technology. Moving forward, embracing collaborative strategies will be essential for driving innovation and ensuring that new medical devices efficiently reach the market, ultimately benefiting patients and healthcare systems worldwide.
Frequently Asked Questions
Why is collaboration important in Medtech trial design?
Collaboration in Medtech trial design is crucial as it unites diverse stakeholders—including researchers, regulatory authorities, and healthcare professionals—towards a shared objective, fostering innovation, enhancing communication, and integrating multiple perspectives into the design process.
What are the benefits of effective teamwork in Medtech studies?
Effective teamwork can expedite the development of medical devices, enhance the quality of data collected, and lead to superior patient outcomes. Trials characterized by robust collaboration achieve higher enrollment rates and improved retention and compliance among participants.
Who are the key stakeholders involved in Medtech trial design?
Key stakeholders include clinical researchers, regulatory agencies, sponsors (such as pharmaceutical corporations or Medtech companies), and patients. Each group contributes uniquely to the study process.
What roles do these stakeholders play in Medtech studies?
Clinical researchers design and conduct studies, regulatory agencies ensure compliance with safety regulations, sponsors provide funding and resources, and patients offer insights into the usability and effectiveness of medical devices.
How does patient involvement impact Medtech studies?
Involving patients early in the study design process leads to more patient-centered outcomes, enhancing recruitment rates and retention. Studies with active patient participation experience increased engagement, which is crucial for research success.
What challenges do small enterprises face in Medtech trial collaboration?
Small enterprises often struggle with managing expenses related to sharing research data, which can hinder partnership and transparency.
What strategies can foster collaboration in Medtech trial design?
Establishing clear communication channels, defining the roles of each stakeholder, and promoting transparency in data sharing are fundamental strategies for fostering collaboration.
Can you provide an example of a successful collaboration in Medtech studies?
An example includes the partnership between bioaccess™ and Caribbean Health Group (CHG) to establish Barranquilla as a significant location for medical studies in Latin America, which aims to attract more research initiatives to the region.
What are the implications of the European Medical Device Regulation on Medtech studies?
The European Medical Device Regulation, effective since 2020, has introduced significant changes affecting how studies are conducted and regulated, emphasizing the need for compliance and safety.
How does bioaccess™ contribute to Medtech trial management?
Bioaccess™ provides extensive management services for studies, including feasibility assessments, site selection, compliance evaluations, project management, and reporting, facilitating smoother trial processes.

