Overview
The article examines best practices for implementing cost-efficient trial strategies within Colombia's clinical research landscape. It asserts that leveraging local resources, conducting early feasibility assessments, ensuring regulatory compliance, embracing technology, and developing targeted recruitment strategies can lead to a substantial reduction in clinical trial costs—ranging from 30% to 75% compared to North America and Western Europe. These strategies not only diminish expenses but also enhance overall research efficiency and outcomes, underscoring their significance in the evolving Medtech environment.
Introduction
In the rapidly evolving landscape of clinical research, Colombia has emerged as a formidable player, particularly in the Medtech sector. This country presents a unique combination of cost-effective healthcare services, a diverse population, and a streamlined regulatory framework, creating an attractive environment for clinical trials. Medtech companies are increasingly drawn to Colombia, where conducting clinical studies can be significantly more affordable—ranging from 30% to 75% less than in North America and Western Europe.
This article delves into the myriad advantages of conducting clinical research in Colombia, emphasizing the importance of:
- Early feasibility studies
- Regulatory compliance
- Innovative recruitment strategies
Furthermore, it highlights the pivotal role of technology in optimizing trial efficiency and ensuring successful outcomes, positioning Colombia as a key destination for Medtech companies aiming to navigate the complexities of clinical trials while maximizing cost-effectiveness.
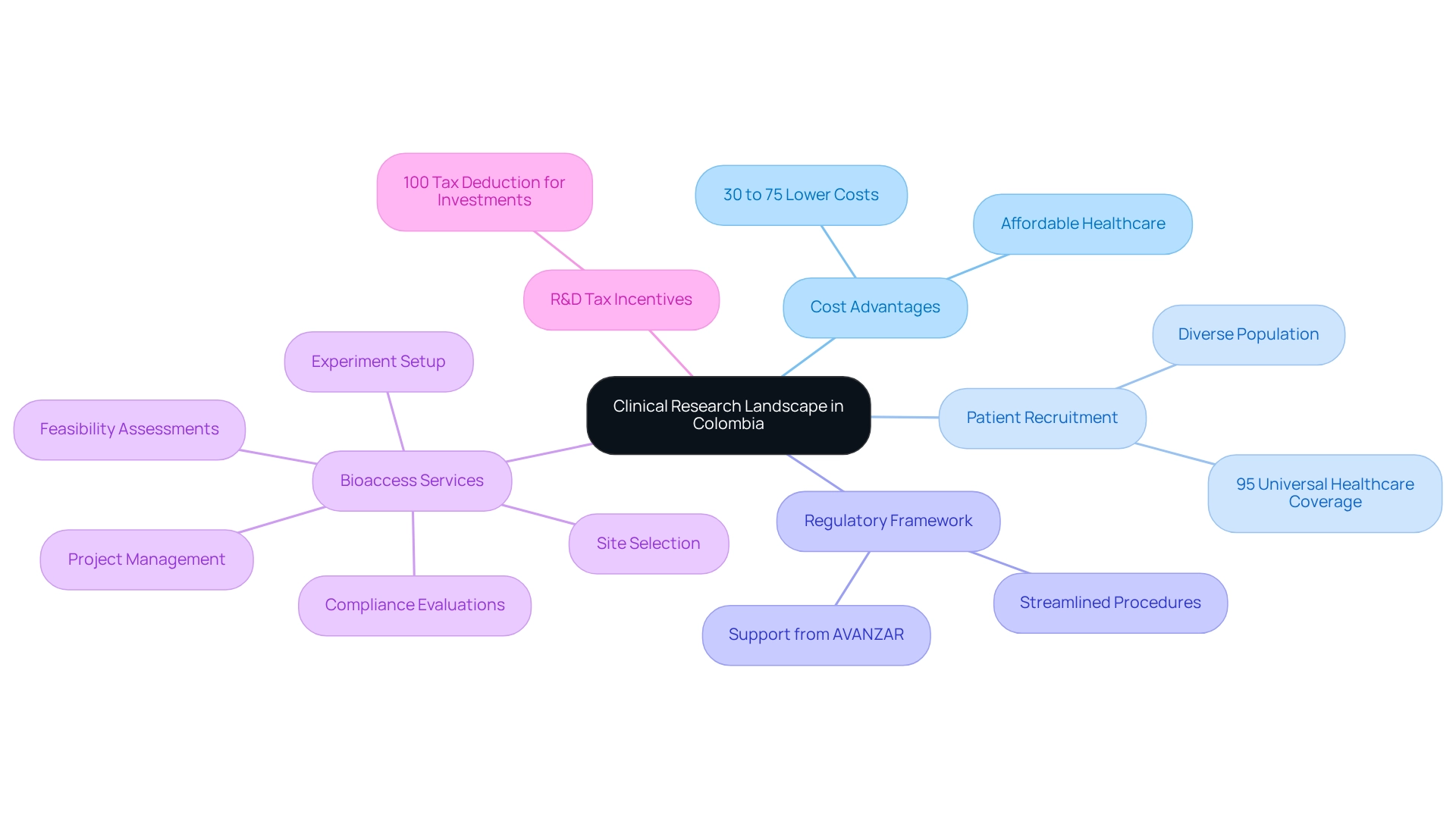
Understanding the Clinical Research Landscape in Colombia
Colombia has established itself as a prominent center for medical research, particularly in the Medtech field, thanks to its affordable healthcare services and a well-organized regulatory framework. Clinical trials conducted in Colombia can be 30% to 75% less expensive than those in North America or Western Europe, using cost-efficient trial strategies tailored for the region. For instance, a professional account for groups of up to five individuals is priced at $1,299 USD, highlighting the cost-effectiveness of research services.
The diverse population of over 50 million not only enriches the patient recruitment environment—where approximately 95% are covered by universal healthcare—but also enhances the overall viability of research trials. Julio G. Martinez-Clark, CEO of bioaccess, emphasizes this point by stating, 'The country's blend of a sizable and varied population, skilled research locations, streamlined regulatory procedures, and a history of successful medtech studies since 2010, along with cost-efficient trial strategies for Colombia, makes it an appealing option for U.S. medical device firms aiming to carry out research.' Furthermore, bioaccess offers extensive services such as feasibility assessments, site selection, compliance evaluations, experiment setup, and project management, all of which improve the clinical research process.
In addition, Colombia provides significant R&D tax incentives, including a 100% tax deduction for investments in science, technology, and innovation projects, further enhancing its appeal for Medtech companies. The case study 'Patient Recruitment Challenges and Opportunities' illustrates the contrasts between patient recruitment in developed nations and the opportunities available in Latin America, where awareness and access to studies are on the rise. As stakeholders navigate this dynamic landscape, understanding these benefits—such as the importance of healthcare facility accreditation and medical professional qualifications—is crucial for optimizing research strategies and capitalizing on the unique opportunities available.
Challenges in Cost Management for Clinical Trials
Carrying out medical studies in Colombia presents a unique set of challenges, despite the region's numerous advantages. As Julio G. Martinez-Clark, CEO of bioaccess Colombia, notes, "The combination of a large and diverse population, experienced research facilities, efficient regulatory processes, a cost-competitive environment, and a history of successful medtech studies since 2010 makes it an appealing option for U.S. medical device companies seeking to conduct research." Navigating the complex regulatory landscape is crucial, as these requirements can lead to significant delays and increased costs.
The regulatory framework demands thorough compliance evaluations and project management, which can strain budgets if not managed effectively. Additionally, logistical hurdles such as site management and patient recruitment introduce another layer of complexity, often exacerbating financial pressures. The impact of these regulatory requirements on research trial budgets is significant. Industry specialists emphasize that the combination of a diverse population and skilled research sites in Colombia offers a promising environment for U.S. medical device firms. However, the associated costs of meeting regulatory standards must be meticulously considered during the planning stages.
Bioaccess® provides comprehensive research project management services, including feasibility assessments and compliance evaluations, to assist sponsors in navigating these challenges effectively. This ensures information security and client trust through robust data protection measures. Furthermore, socio-economic factors in the region can lead to ethical dilemmas for sponsors. Many patients may view participation in research studies as their only treatment option, raising concerns about informed consent and patient vulnerability. This highlights the necessity of collaborating closely with local investigators to uphold ethical practices and protect patient rights.
As outlined in a case analysis regarding socio-economic factors influencing medical research, ongoing initiatives to enhance healthcare coverage are essential, yet challenges remain. Sponsors must work alongside local investigators to maintain ethical standards, supported by bioaccess®'s grievance and data protection procedures that ensure client concerns are addressed with compliance and transparency. As the landscape evolves in 2025, the proactive engagement of ethics committees will be vital in safeguarding participants against exploitation and bias.
The partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a key hub for medical studies in Latin America, further enhancing the region's appeal. By understanding these challenges and devising cost-efficient trial strategies tailored for Colombia, research sponsors can improve the financial viability of their projects while upholding high standards of quality and compliance.
Leveraging Early Feasibility Studies for Cost Efficiency
Initial feasibility assessments (EFS) play a pivotal role in evaluating the viability of clinical experiments prior to their comprehensive implementation. By conducting EFS, researchers gather essential preliminary data concerning safety and efficacy, which is vital for refining study designs and protocols. This proactive strategy mitigates the risk of failures and facilitates optimal resource allocation, leading to substantial cost savings.
Organizations that adopt EFS can enhance their overall evaluation processes, exemplifying cost-efficient trial strategies for Colombia. This practice stands as a best practice for Medtech companies operating in the region. The correlation coefficient of 0.500, accompanied by a 95% confidence interval of (0.170, 0.729), highlights the effectiveness of EFS in improving trial outcomes.
The advantages of EFS extend beyond mere cost efficiency; they also foster collaboration among stakeholders. For instance, the case analysis titled 'Training and Education for EFS Stakeholders' illustrates how developing targeted training and educational resources for EFS participants can significantly improve adherence to regulatory requirements and deepen understanding of the EFS process. This collaborative approach not only bolsters the credibility of the research but also nurtures a culture of continuous improvement within the research environment.
As the Medtech sector evolves, the importance of EFS in research studies becomes increasingly evident. Jeffrey Shuren, M.D., J.D., Director of the Center for Devices and Radiological Health, underscores this by stating, "Continuing America’s leadership: the future of medical innovation for patients." By leveraging preliminary viability assessments, companies can reduce the likelihood of costly testing failures and expedite the path to market for innovative medical devices.
Moreover, the FDA CDRH EFS program seeks to enhance early access to beneficial medical devices, establishing a standardized process for conducting U.S. EFS. This strategic focus on EFS not only aligns with industry goals but also empowers organizations like bioaccess®, which boasts over 20 years of experience in Medtech, to explore cost-efficient trial strategies for Colombia and tap into the untapped potential of conducting research in Latin America. Furthermore, bioaccess® manages a diverse array of studies, including First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF), ensuring a tailored approach to navigating research in the region.
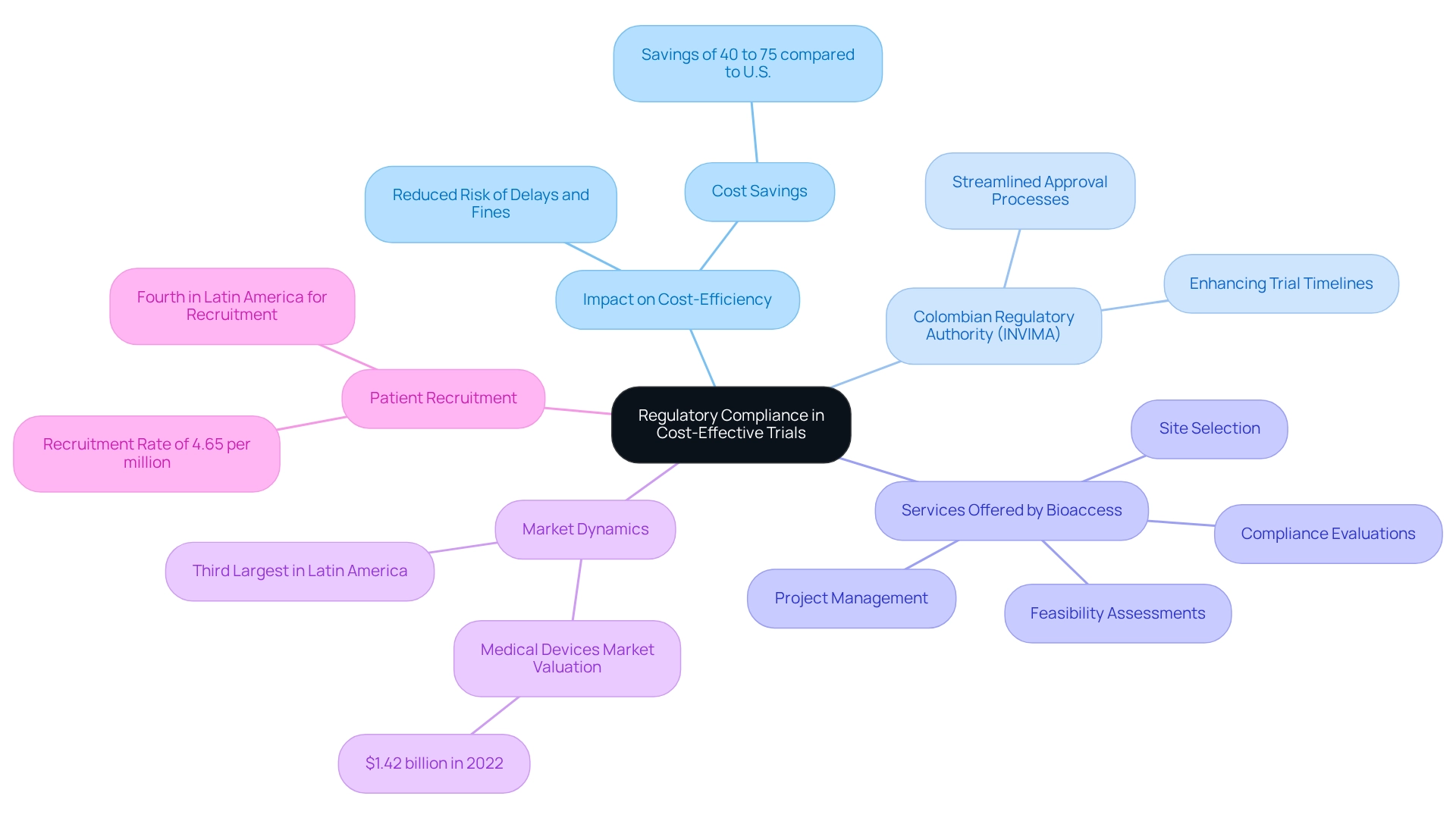
The Role of Regulatory Compliance in Cost-Effective Trials
Regulatory compliance is often perceived as a burdensome necessity; however, it plays a critical role in enhancing the cost-efficiency of medical studies. By adhering to established protocols and guidelines from the outset, companies can significantly reduce the risk of costly delays and fines associated with non-compliance. In Colombia, the regulatory authority INVIMA has made notable advancements in streamlining approval processes, which not only accelerates timelines but also contributes to implementing cost-efficient trial strategies for the country.
As emphasized by MarkWide Research, their reports are detailed, precise, and delivered punctually, highlighting the importance of reliable information in managing these procedures. Bioaccess offers comprehensive management services for studies, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project management
- Reporting
Investing in compliance safeguards the integrity of clinical trials and fosters trust among stakeholders, which is essential for successful outcomes. Notably, the medical devices market in Colombia was valued at $1.42 billion in 2022, establishing it as the third-largest in Latin America.
This robust market, coupled with INVIMA's effective regulatory framework, creates a promising environment for conducting trials. Furthermore, data reveals that Colombia ranks fourth in Latin America for recruiting and not-yet recruiting research per million individuals, with a recruitment rate of 4.65. This strong capacity for patient recruitment is vital for the success of clinical trials, as it directly impacts trial efficiency and costs.
A case analysis illustrates that the expenses associated with experimental medical treatments in Colombia can be significantly lower than those in the U.S., with savings ranging from 40% to 75%. Implementing cost-efficient trial strategies for Colombia fosters an environment conducive to more comprehensive and detailed studies, benefiting both patients and the advancement of medical knowledge without compromising quality.
Ultimately, understanding local market dynamics and regulatory compliance is essential for companies aiming to thrive in the evolving Medtech landscape of the region. By leveraging the right collaborations and tailored approaches, organizations can unlock substantial opportunities for growth and innovation while employing cost-efficient trial strategies in Colombia.
Harnessing Technology to Optimize Trial Efficiency
Technology stands as a cornerstone in enhancing clinical studies, particularly within the dynamic landscape of Colombia's Medtech sector. The implementation of cost-efficient trial strategies, such as electronic data capture (EDC) systems, remote monitoring tools, and patient engagement platforms, has proven to significantly lower operational costs while enhancing data accuracy. For instance, the incorporation of telemedicine for patient follow-ups not only reduces travel expenses but also improves participant retention rates, a critical element for study success.
Moreover, advanced analytics play a vital role in identifying trends and optimizing decision-making processes, resulting in more efficient test management. A notable case study involves bioaccess®, which boasts over 20 years of experience in managing diverse studies, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies. Their collaboration with Caribbean Health Group aims to position Barranquilla as a leading site for medical studies in Latin America, supported by Colombia's Minister of Health.
This initiative is poised to create jobs, stimulate economic growth, and enhance healthcare outcomes in the region.
As Max Baumann, Head of Execution at Treehill Partners, articulates, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success." Embracing these technological advancements is crucial for Medtech firms pursuing cost-efficient trial strategies in Colombia. The industry increasingly prioritizes patient engagement and feedback, which not only improves outcomes but also aligns with the growing emphasis on optimizing development journeys.
By 2025, the focus on technology implementation in medical studies is expected to yield significant cost savings, underscoring the importance of cost-efficient trial strategies for Colombia as a vital sector for strategic investment.
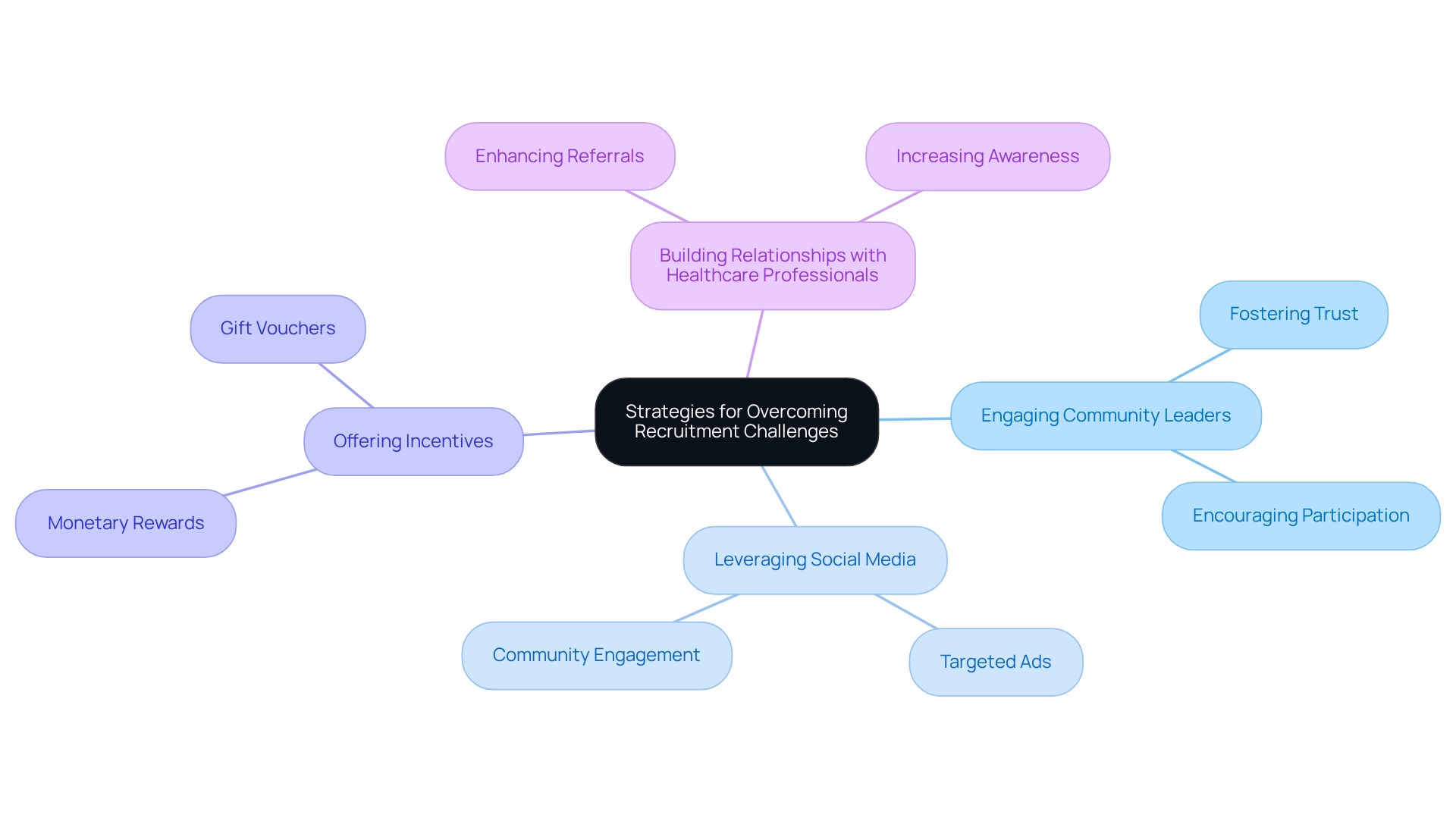
Strategies for Overcoming Recruitment Challenges
Recruitment difficulties often impede the advancement of research studies, leading to postponements and increased expenses. To effectively navigate these obstacles, it is crucial to adopt targeted recruitment strategies tailored to the local demographic. Engaging with community leaders fosters trust and encourages participation, while leveraging social media platforms enhances outreach and visibility.
Additionally, offering incentives for participation can motivate potential subjects to enroll. Building strong connections with healthcare professionals is essential, as these relationships aid in referrals and enhance awareness about ongoing studies. By focusing on cost-efficient trial strategies for Colombia, Medtech companies can achieve timely participant enrollment, thereby reducing costs linked to delays and improving the overall efficiency of trials.
In this South American country, the landscape for clinical trials is particularly promising due to its large population of over 50 million and efficient regulatory processes, with the INVIMA overseeing medical device compliance as a Level 4 health authority. Successful case examples, such as the partnership between bioaccess® and Caribbean Health Group, have established Barranquilla as a premier location for medical research. This partnership demonstrates that innovative recruitment strategies can lead to significant improvements in participant engagement.
For instance, GlobalCare Clinical Trials reported over a 50% reduction in recruitment duration and impressive retention rates of 95%. As Max Baumann, Head of Execution, noted, "Going into 2025, we still notice biotech confronting essential business model obstacles as end-markets become increasingly crowded." Moreover, the support services market for medical research in the country is expected to grow considerably by 2030, presenting a significant opportunity for Medtech firms to utilize cost-efficient trial strategies for Colombia.
With over 20 years of expertise in the Medtech field, bioaccess® is well-positioned to guide companies through these evolving challenges. bioaccess® specializes in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). Furthermore, Colombia provides R&D tax and financial incentives, including a 100% tax deduction for investments in science, technology, and innovation projects, making it a compelling location for trials.
Importance of Post-Market Clinical Follow-Up in Cost Management
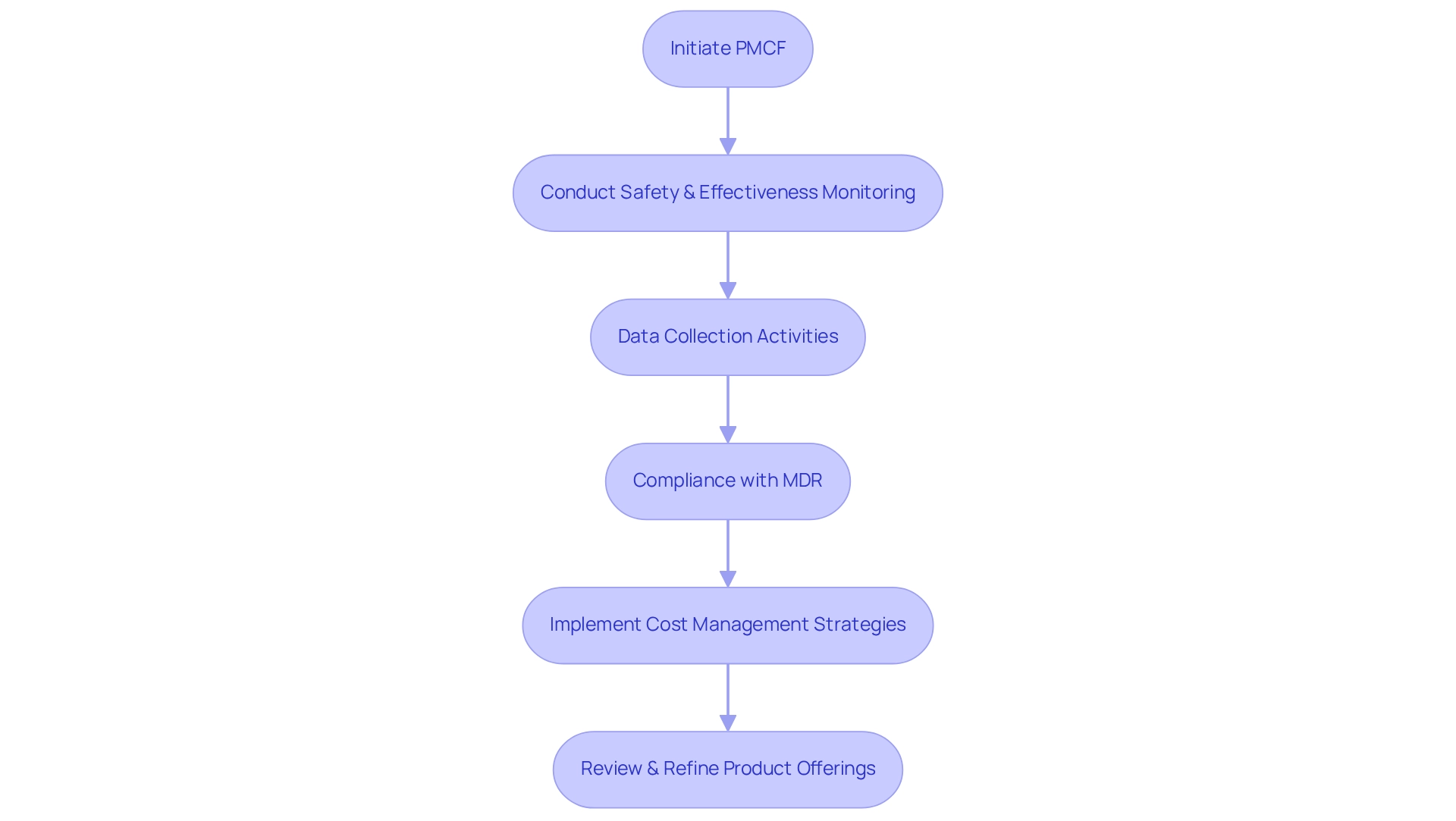
Post-market clinical follow-up (PMCF) evaluations are crucial for monitoring the long-term safety and effectiveness of medical devices once they reach the market. These investigations yield vital insights that not only inform future product enhancements but also ensure compliance with regulatory standards, thereby mitigating costs associated with recalls or adverse incidents. By proactively engaging in PMCF, Medtech companies can refine their product offerings and maintain a competitive edge in the marketplace.
As we approach 2025, the importance of PMCF assessments is underscored by the need for comprehensive plans that adhere to the Medical Device Regulation (MDR) requirements. These plans are integral to the overall post-market surveillance (PMS) strategy and technical documentation. They must articulate data collection activities, objectives, scientific rationale, and timelines—elements that are essential for effective post-market surveillance. bioaccess® utilizes its expertise in managing PMCF studies, along with Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Pilot Studies, to streamline these processes, ensuring that manufacturers can efficiently gather data while complying with regulations like HIPAA and GDPR.
Expert opinions assert that through PMCF programs, the medical device sector can ensure that products consistently meet the highest standards of safety and performance, adapting to evolving medical practices and patient needs. As Adi Ickowicz, Senior Principal at the MedTech Unit of Asphalion S.L., articulates, "Through PMCF programs, the medical device sector guarantees that its products persist in meeting the highest standards of safety and performance amidst constantly evolving medical practices and patient requirements." This proactive approach not only bolsters regulatory compliance but also facilitates the adoption of cost-efficient trial strategies for Colombia, resulting in substantial long-term cost savings.
Furthermore, the intended application of the device and data consent under GDPR significantly impacts data collection capabilities, a critical factor for effective PMCF research. By integrating PMCF into their overarching healthcare strategy, Medtech companies can achieve sustainable cost management while implementing economical trial strategies for Colombia, thereby enhancing product quality and safety. Additionally, maintaining Design History Files (DHF) as mandated by 21 CFR part 820 of the FDA is vital for ensuring compliance and supporting the integrity of PMCF studies.
With over 20 years of experience in Medtech, bioaccess® is exceptionally equipped to navigate these complexities and advocate for cost-efficient trial strategies for Colombia in research studies.
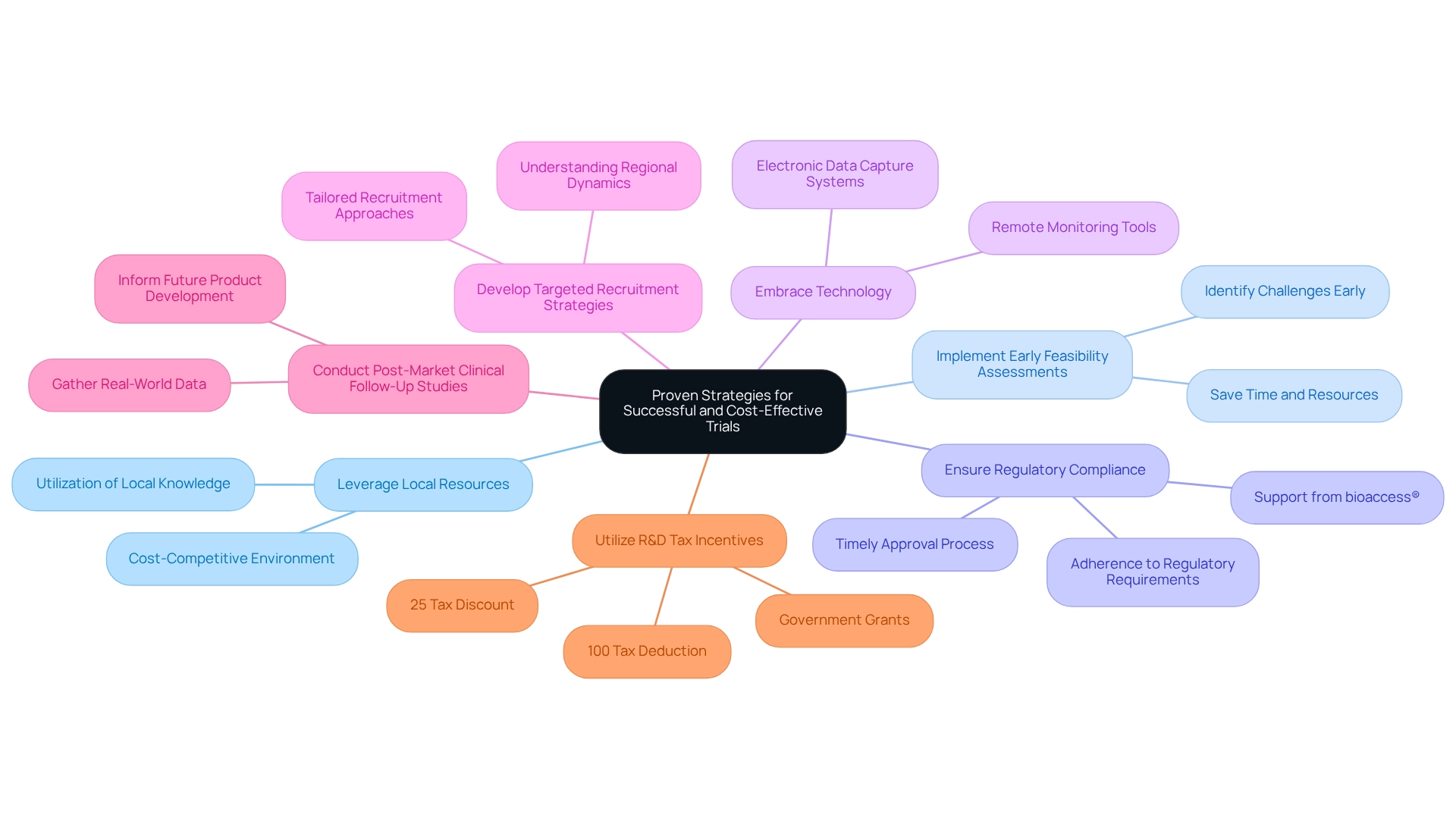
Proven Strategies for Successful and Cost-Effective Trials
To achieve successful and cost-effective clinical trials in Colombia, stakeholders should focus on several key strategies.
-
Leverage Local Resources: Colombia offers a cost-competitive environment where medical procedures can be 30%–75% less expensive than in North America or Western Europe. By employing local knowledge and resources, stakeholders can significantly reduce testing expenses while aligning with cost-efficient trial strategies that uphold high-quality standards. Julio G. Martinez-Clark, CEO of bioaccess®, emphasizes that "the nation’s combination of a large and diverse population, skilled research sites, and efficient regulatory processes make it an appealing choice for U.S. medical device companies."
-
Implement Early Feasibility Assessments: Conducting early feasibility assessments allows companies to identify potential challenges before committing to full-scale trials. This proactive approach is essential for implementing cost-efficient trial strategies in Colombia, ultimately saving time and resources through early issue identification.
-
Ensure Regulatory Compliance: Strict adherence to regulatory requirements is crucial to avoid costly delays. The effective regulatory processes in Colombia, supported by organizations such as AVANZAR, facilitate prompt approvals that enhance the efficiency of research studies. For instance, bioaccess® assists companies in navigating the regulatory landscape by providing support in classification, documentation preparation, and communication with ethics committees, ensuring a timely approval process for medical devices. Notably, the total IRB/EC and MoH (INVIMA) review in Colombia typically takes only 90-120 days.
-
Embrace Technology: Utilizing advanced technology can streamline clinical study processes and enhance data management. Implementing electronic data capture systems and remote monitoring tools not only improves accuracy but also reduces administrative burdens.
-
Develop Targeted Recruitment Strategies: Understanding regional market dynamics is essential for effective recruitment. Tailoring recruitment strategies to Colombia's local population, which exceeds 50 million and is approximately 95% covered by universal healthcare, ensures timely participant enrollment—critical for maintaining trial timelines.
-
Conduct Post-Market Clinical Follow-Up Studies: Gathering real-world data through post-market studies provides valuable insights that inform future product development and enhance understanding of a device's performance across diverse populations.
-
Utilize R&D Tax Incentives: Companies can capitalize on significant R&D tax incentives in Colombia, including a 100% tax deduction, a 25% tax discount, and a 50% future tax credit, along with approximately $10 million in government grants for science, technology, and innovation projects.
As Latin America's third-largest medical device market, Colombia represents a pivotal area for conducting clinical studies. By implementing these cost-efficient trial strategies, Medtech firms can enhance their research processes in the region, leading to improved outcomes and a more effective route to market. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, underscores the significance of these strategies, drawing from his experience with bioaccess® during its initial human study in South America.
Furthermore, the World Health Organization ranks Colombia's healthcare system as #22 globally, further underscoring the quality of care available for clinical trials.
Conclusion
The clinical research landscape in Colombia presents a wealth of opportunities for Medtech companies seeking to conduct trials in a cost-effective manner. By leveraging the country's affordable healthcare services, diverse population, and streamlined regulatory framework, organizations can significantly reduce trial costs—ranging from 30% to 75% compared to North America and Western Europe. The importance of early feasibility studies, regulatory compliance, and innovative recruitment strategies cannot be overstated, as these elements are pivotal in optimizing trial efficiency and ensuring successful outcomes.
Moreover, the integration of advanced technology plays a crucial role in enhancing data accuracy and participant engagement, further driving down operational costs. With a robust market for medical devices and favorable R&D tax incentives, Colombia stands out as a prime destination for clinical trials, offering a unique blend of quality and affordability.
As stakeholders navigate the complexities of clinical research, understanding and addressing the challenges inherent in this landscape—such as regulatory compliance and ethical considerations—will be vital. By adopting proven strategies and harnessing local resources, Medtech companies can not only streamline their trial processes but also contribute to the advancement of medical knowledge and innovation in the region.
In conclusion, Colombia's evolving Medtech sector offers a compelling case for clinical research, marked by significant cost savings and a commitment to quality. By embracing the strategies outlined, companies can position themselves for success, ensuring that they remain competitive and capable of delivering innovative medical solutions to market effectively. The time to explore the opportunities within Colombia's clinical research environment is now, as it promises to be a game-changer for the Medtech industry.
Frequently Asked Questions
Why is Colombia considered a prominent center for medical research, particularly in the Medtech field?
Colombia is recognized for its affordable healthcare services and a well-organized regulatory framework, making clinical trials significantly less expensive—30% to 75% lower than in North America or Western Europe.
What are the cost advantages of conducting clinical trials in Colombia?
Clinical trials in Colombia can be cost-efficient, with professional account services priced at $1,299 USD for groups of up to five individuals, showcasing the overall affordability of research services.
How does Colombia's population benefit medical research?
Colombia has a diverse population of over 50 million, which enriches the patient recruitment environment. Approximately 95% of the population is covered by universal healthcare, enhancing the viability of research trials.
What services does bioaccess provide to support clinical research in Colombia?
Bioaccess offers a range of services including feasibility assessments, site selection, compliance evaluations, experiment setup, and project management to improve the clinical research process.
What tax incentives does Colombia offer to Medtech companies?
Colombia provides significant R&D tax incentives, including a 100% tax deduction for investments in science, technology, and innovation projects, which enhances its appeal for Medtech firms.
What challenges do researchers face when conducting studies in Colombia?
Researchers encounter challenges such as navigating a complex regulatory landscape, logistical issues like site management and patient recruitment, and socio-economic factors that can lead to ethical dilemmas.
How does bioaccess assist sponsors in navigating the challenges of medical research in Colombia?
Bioaccess provides comprehensive research project management services, including compliance evaluations and feasibility assessments, to help sponsors effectively navigate regulatory challenges and ensure data protection.
Why is ethical consideration important in medical research in Colombia?
Ethical considerations are crucial due to socio-economic factors that may lead patients to view participation in studies as their only treatment option, raising concerns about informed consent and patient vulnerability.
What role do ethics committees play in the evolving research landscape in Colombia?
Proactive engagement of ethics committees will be vital in safeguarding participants against exploitation and bias as the research landscape evolves in 2025.
What initiative is being taken to enhance Barranquilla's position in medical studies?
The partnership between bioaccess and Caribbean Health Group aims to establish Barranquilla as a key hub for medical studies in Latin America, enhancing the region's appeal for research.




