Overview
This article highlights best practices for selecting a Contract Research Organization (CRO) in Latin America, emphasizing its critical role in ensuring quality and efficiency in clinical trials. The selection of the right CRO is paramount for optimizing study outcomes; a well-chosen CRO can significantly enhance patient recruitment, ensure regulatory compliance, and ultimately reduce costs and timelines.
Supported by examples of successful partnerships, the growing advantages of conducting research in this region are evident. As the Medtech landscape evolves, understanding the strategic value of CROs becomes increasingly essential for clinical research success.
Introduction
In the rapidly evolving landscape of clinical research, the selection of an appropriate Contract Research Organization (CRO) in Latin America has become a pivotal factor in determining the success of clinical trials. Given the region's unique regulatory environments, diverse patient demographics, and inherent cost advantages, the choice of a CRO can profoundly impact timelines, costs, and the quality of clinical data.
As the Medtech sector continues to expand, it is essential for sponsors to understand the key criteria for evaluating CROs to effectively navigate this complex terrain. Insights from industry leaders highlight the significance of regulatory compliance and effective communication strategies, underscoring the necessity of a strategic partnership in achieving successful clinical outcomes.
With Latin America emerging as a compelling destination for clinical trials, the implications of CRO selection are more crucial than ever.
The Significance of Choosing the Right CRO in Latin America
The selection of a suitable Contract Research Organization (CRO) is crucial for the success of medical studies, particularly in the context of Latin America. A strategically chosen CRO can significantly streamline processes, enhance patient recruitment, and ensure compliance with regulatory standards. The right partner is essential for Latin America CRO selection, as they not only bring specialized expertise but also possess invaluable local knowledge, which is crucial for navigating the complex and varied regulatory landscapes across different Latin American countries.
This decision directly influences timelines, costs, and ultimately, the quality of the data generated. In 2025, the influence of CRO selection on research success rates is more pronounced than ever. Studies indicate that a well-chosen CRO can enhance the likelihood of achieving study objectives, thereby reducing the average cost of bringing a drug to market, which can exceed $1 billion. By effectively managing these aspects, a capable CRO can help reduce costs and enhance overall efficiency in the research process.
Furthermore, the importance of selecting the right CRO is underscored by successful collaborations—such as those between bioaccess® and Caribbean Health Group, and bioaccess® with Welwaze Medical Inc. for the Celbrea® launch—which can lead to improved patient outcomes and faster market access for innovative medical devices. Bioaccess® provides an extensive array of solutions customized for Medtech companies, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Pilot studies
- First-in-human studies
- Pivotal studies
- Post-market research follow-up studies
These services position bioaccess® as a valuable partner in the region, capable of addressing the unique challenges faced by Medtech startups. Their recent partnership with GlobalCare Clinical Trials to enhance ambulatory services in Colombia exemplifies their commitment to efficiency, achieving over 50% reduction in recruitment time and 95% retention rates.
A client from GlobalCare remarked, "Our partnership with bioaccess® has revolutionized our method for studies, enabling us to attain unmatched efficiency and patient involvement."
As the Medtech sector continues to evolve, the significance of Latin America CRO selection for trials cannot be overstated. Expert opinions underscore that local knowledge and a customized strategy are essential for addressing the distinct challenges of the area, ultimately resulting in higher success rates in research studies. Conner Riley, a consultant at You Clinical Research group, states, "Utilizing our 30 years of industry experience, You is able to provide comprehensive staffing solutions to clients of all sizes."
By leveraging the strengths of an effective CRO like bioaccess®, companies can ensure that their studies are not only efficient but also produce high-quality data that supports regulatory approval and market entry.
Why Latin America is an Attractive Destination for Clinical Trials
The region of America presents numerous advantages for research studies, making it an increasingly attractive location for sponsors. It boasts a diverse and urbanized population, which is essential for studies requiring varied patient demographics. This genetic diversity enriches the research landscape and enhances the applicability of findings across different populations.
Cost-effectiveness stands out as another significant advantage of selecting Latin America CROs for research studies. Research indicates that expenses linked to medical studies in this region can be up to 50% lower than those in North America and Europe. This substantial difference enables sponsors to allocate their research budgets more efficiently, facilitating the advancement of medical technologies without compromising quality.
Moreover, comprehensive research study management services—such as feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting of adverse events—are crucial for successful research. For instance, bioaccess provides robust support in these areas, ensuring that evaluations comply with local regulations and standards set by the Colombia National Food and Drug Surveillance Institute (INVIMA), recognized as a Level 4 health authority by PAHO/WHO.
The regulatory landscape in South America is becoming increasingly favorable for medical research. Countries in the region are streamlining their approval procedures and adopting more flexible regulations, which can expedite the initiation of research studies. Specifically, the total IRB/EC and MoH (INVIMA) review in Colombia typically takes 90-120 days.
This shift not only enhances the speed of research but also improves the Latin America CRO selection, positioning the region as a key player in the global medical research arena.
A compelling statistic underscores the benefits of conducting research studies in this area: dropout rates in South America are one-third of those in the U.S. and the EU. This notably reduced dropout rate enhances the reliability of research outcomes and further solidifies the region's status as a viable choice for sponsors.
Additionally, addressing current challenges is vital for the region to emerge as a significant participant in the global research landscape. A case study highlights the importance of overcoming language barriers in medical studies conducted in Latin America. Language differences can complicate the logistics of experiments, particularly when studies originate from outside the region.
By employing culturally sensitive translation methods and understanding local dialects, organizations can significantly improve the informed consent process, thereby enhancing the overall quality of research studies. The results of such efforts demonstrate that addressing language barriers can prevent misunderstandings and boost participant engagement.
Furthermore, R&D tax incentives provide additional motivation for sponsors to conduct studies in Colombia. Investments in science, technology, and innovation projects qualify for a 100% tax deduction, a 25% tax discount, a 50% future tax credit, and approximately $10 million in government grants.
In summary, the combination of a diverse population, cost benefits, and a transforming regulatory environment positions the region as an ideal site for Latin America CRO selection for research studies in 2025. These factors not only enhance the efficiency of research but also support broader economic development and healthcare improvements in the region. As Russ Greenspan, CTO of PresenceLearning, remarked, "This alignment has been crucial in maintaining productivity and efficiency," underscoring the significance of collaboration and understanding in research.
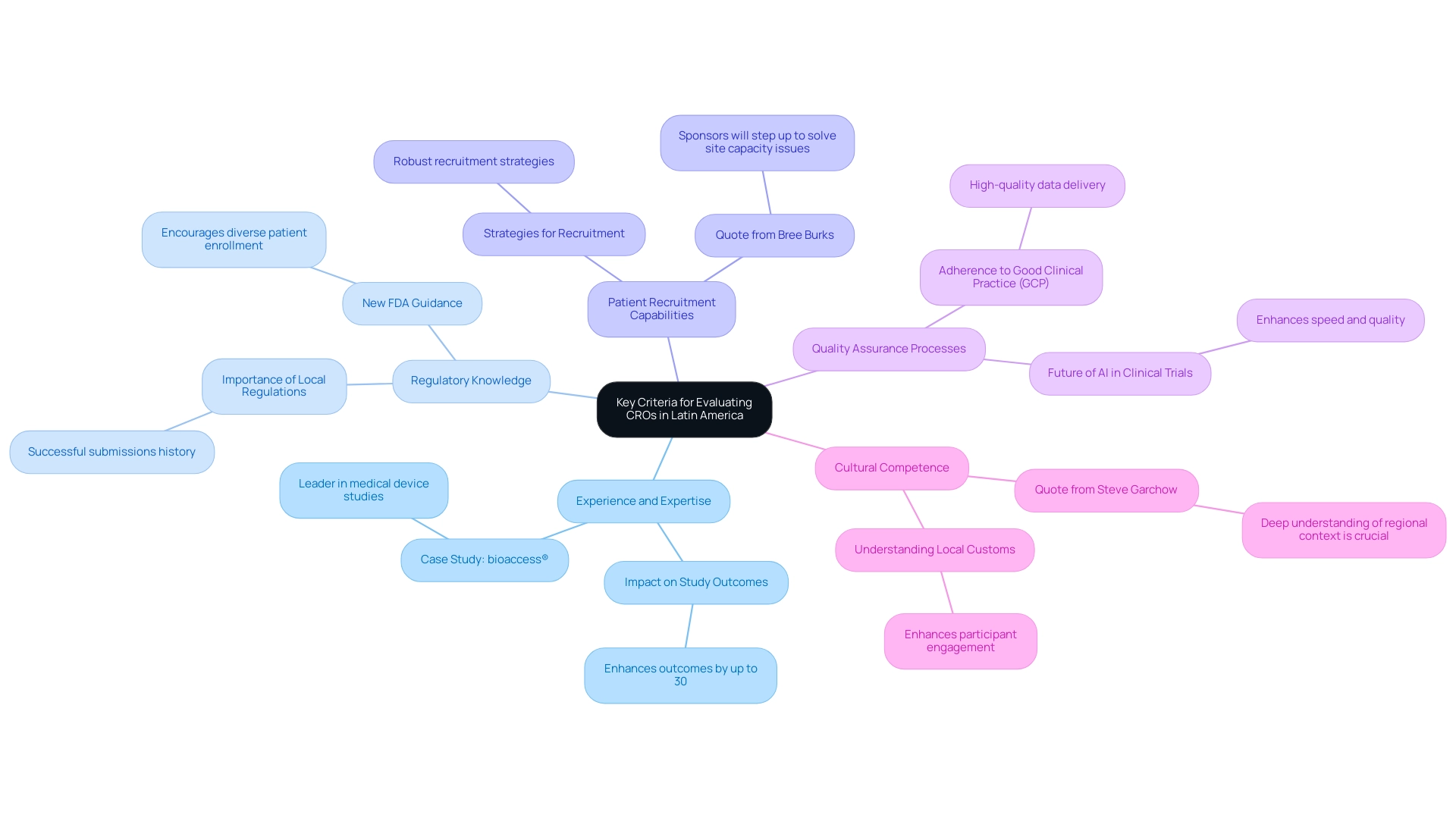
Key Criteria for Evaluating CROs in Latin America
When selecting a contract research organization (CRO) in Latin America, it is essential to evaluate several key criteria to ensure successful clinical trial outcomes:
- Experience and Expertise: Examine the CRO's history and performance in your specific therapeutic area. A CRO with a proven history can significantly impact the success of your studies, as research indicates that experienced CROs can enhance study outcomes by up to 30%. For example, bioaccess® has positioned itself as a leader in supporting medical device clinical studies, utilizing extensive knowledge in the South American market to improve study success. Dushyanth Surakanti, CEO of Sparta Biomedical, noted that bioaccess® played a crucial role during their first human study in Colombia, demonstrating their expertise in navigating local challenges.
- Regulatory Knowledge: It is crucial that the CRO possesses in-depth knowledge of local regulations and has a history of successful submissions. In 2025, the importance of regulatory expertise cannot be overstated, as new FDA guidance encourages the enrollment of diverse patient populations, necessitating adept navigation of regulatory landscapes. Steve Garchow highlights the importance of this knowledge in ensuring smooth project execution, which is essential for effective management and Latin America CRO selection in various countries.
- Patient Recruitment Capabilities: Assess the CRO's strategies for patient recruitment and retention. Effective recruitment is essential, as delays in patient enrollment can prolong study timelines and raise expenses. Bree Burks, Vice President of Site Strategy at Veeva, highlights that "Sponsors will step up to solve site capacity issues," underscoring the necessity for CROs to have robust recruitment strategies. Dushyanth Surakanti shared how bioaccess® effectively navigated patient recruitment during their first human study in Colombia, showcasing their capabilities in this area and the importance of understanding local patient dynamics.
- Quality Assurance Processes: Investigate the CRO's quality management systems and adherence to Good Clinical Practice (GCP). High-quality data is paramount, and CROs that implement stringent quality assurance processes are more likely to deliver reliable results. This is increasingly important as AI and machine learning technologies are set to enhance clinical study processes in 2025, ensuring consistent data quality and enabling the development of novel digital biomarkers.
- Cultural Competence: Understanding local customs and practices can significantly enhance execution. CROs that exhibit cultural competence, like bioaccess®, are better positioned to engage with participants and stakeholders, fostering trust and enhancing overall study performance. As Steve Garchow noted, a deep understanding of the regional context is crucial for effective collaboration and market access. This cultural awareness helps in tailoring approaches that resonate with local populations.
By thoughtfully evaluating these criteria and utilizing insights from leaders such as Dushyanth Surakanti and Steve Garchow, sponsors can make a Latin America CRO selection that not only fulfills their specific requirements but also aids in the successful progress of medical devices in the American market. The experiences shared by industry leaders serve as valuable insights for navigating this complex landscape, emphasizing the importance of a structured approach to CRO selection.
Ensuring Regulatory Compliance and Quality Assurance in CRO Selection
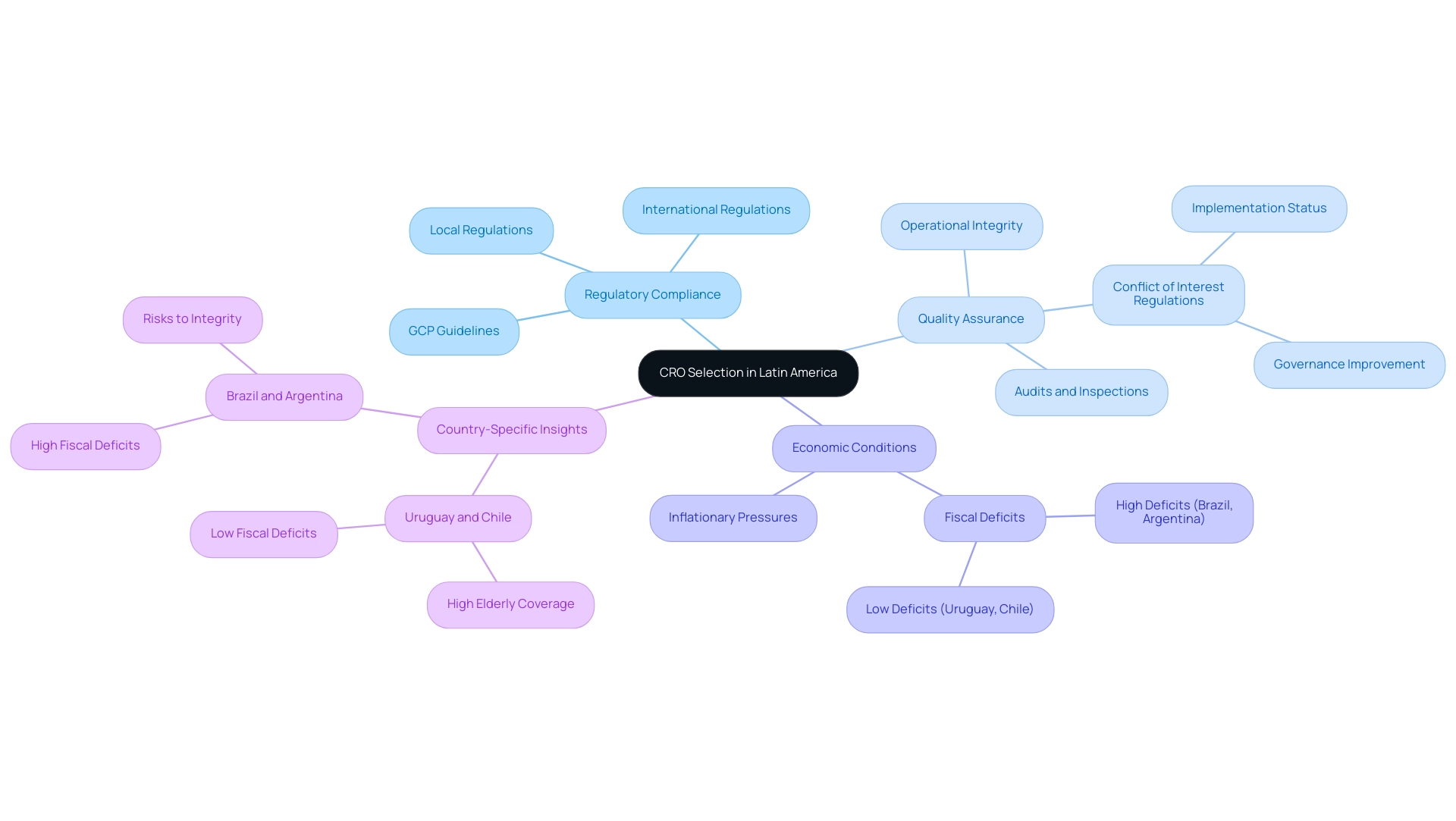
Regulatory adherence and quality control are paramount in the selection of Contract Research Organizations (CROs) in Latin America for research studies. Ensuring that a CRO complies with both local and international regulations, including ethical standards and Good Clinical Practice (GCP) guidelines, is crucial. In 2025, the selection of CROs in Latin America will significantly influence study outcomes, as adherence to GCP among regional CROs is projected to reflect their commitment to high-quality research.
At bioaccess®, our comprehensive clinical study management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Reporting on study status
- Inventory management of serious and non-serious adverse events
To assess a CRO's dedication to quality, conducting thorough audits and reviewing past inspection reports can provide valuable insights into their operational integrity. Our robust quality assurance framework guarantees that we uphold high standards throughout the testing process, safeguarding the integrity of the data collected.
For instance, the implementation of conflict of interest regulations across South America highlights the need for effective governance; while 93% of countries have regulations in place, only one-third enforce them effectively. This discrepancy underscores the importance of selecting a CRO in Latin America that not only complies with regulations but also demonstrates a commitment to ethical practices. Furthermore, the significance of regulatory compliance in CRO selection cannot be overstated. Countries like Uruguay and Chile, with low fiscal deficits and high elderly population coverage, enhance the reliability of CROs operating within these regions.
Conversely, nations facing high fiscal deficits, such as Brazil and Argentina, may present additional risks that could affect the integrity of the process. As noted by Daniel Gonzalez, inflationary pressures in certain American countries could further complicate the economic landscape, influencing the monetary policies that affect CRO operations. Consequently, the selection process for CROs in Latin America must prioritize organizations with a demonstrated history of regulatory adherence and quality assurance, like bioaccess, to guarantee successful research studies in South America.
Additionally, understanding the competitive landscape, where the healthcare CRO industry in the U.S. held the largest share in North America in 2024, provides valuable context for the selection of CROs in Latin America.
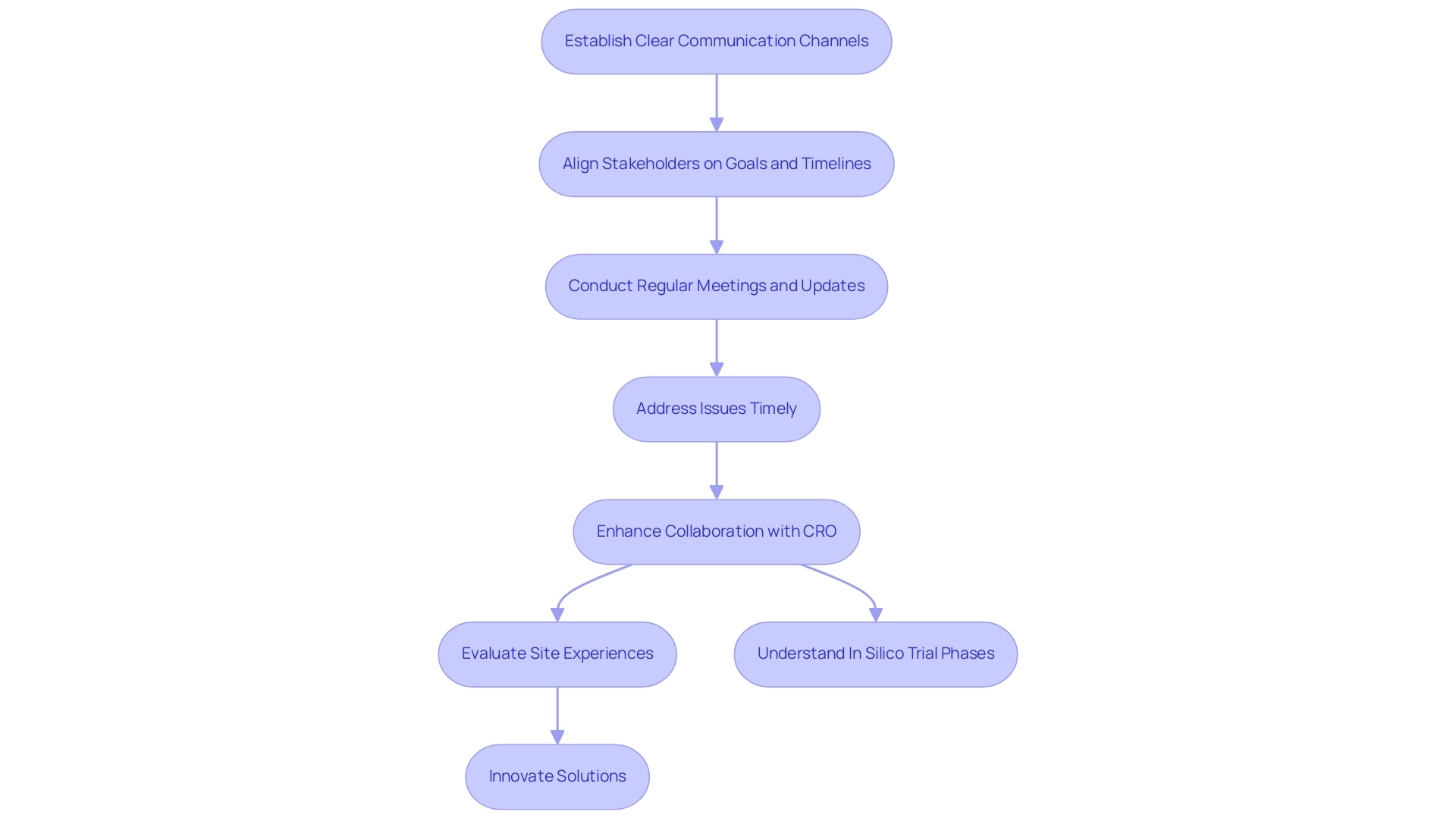
Fostering Effective Communication and Collaboration with Your CRO
Effective communication and collaboration with your Latin America CRO selection are paramount for achieving successful research outcomes. From the outset, it is essential to establish clear communication channels that align all stakeholders on project goals and timelines. Regular meetings and updates facilitate the timely resolution of issues and help maintain momentum throughout the process.
As Saisuman Revankar observes, clear communication is critical for navigating the complexities of clinical studies, ensuring that all parties remain aligned. The significance of collaboration cannot be overstated; research indicates that partnerships between sponsors and CROs—especially in the context of Latin America CRO selection—that emphasize open communication experience higher success rates in study completion. For instance, companies like Alcon have demonstrated the advantages of evaluating site experiences through monitoring data entry times and simplifying query responses.
Such innovations not only alleviate the burden on sites but also enhance patient care, ultimately improving study efficiency. This case illustrates how effective collaboration can lead to tangible enhancements in legal processes, contributing to local economies through job creation and advancements in healthcare.
In 2025, statistics reveal that effective communication strategies can yield a 30% increase in success rates. Furthermore, understanding the phases of in silico trials—phases I, II, III, and IV—is essential in the context of CRO collaboration. By nurturing a collaborative partnership with your CRO, particularly in the context of Latin America CRO selection, you can enhance problem-solving capabilities and foster innovative solutions that benefit the study.
This approach not only simplifies procedures but also ensures that all stakeholders are engaged and committed to the study's success, paving the way for more effective and efficient medical research. Moreover, thorough MDR solutions that encompass study design, data gathering, analysis, and submission highlight the intricacies involved in research studies and the essential role CROs play in overseeing these processes. Understanding the segmentation of the in silico research market can also provide valuable insights into the industry landscape, further guiding your selection process for Latin America CRO.
Import permits and the nationalization of investigational devices are also critical components of the setup that facilitate compliance and expedite the research process.
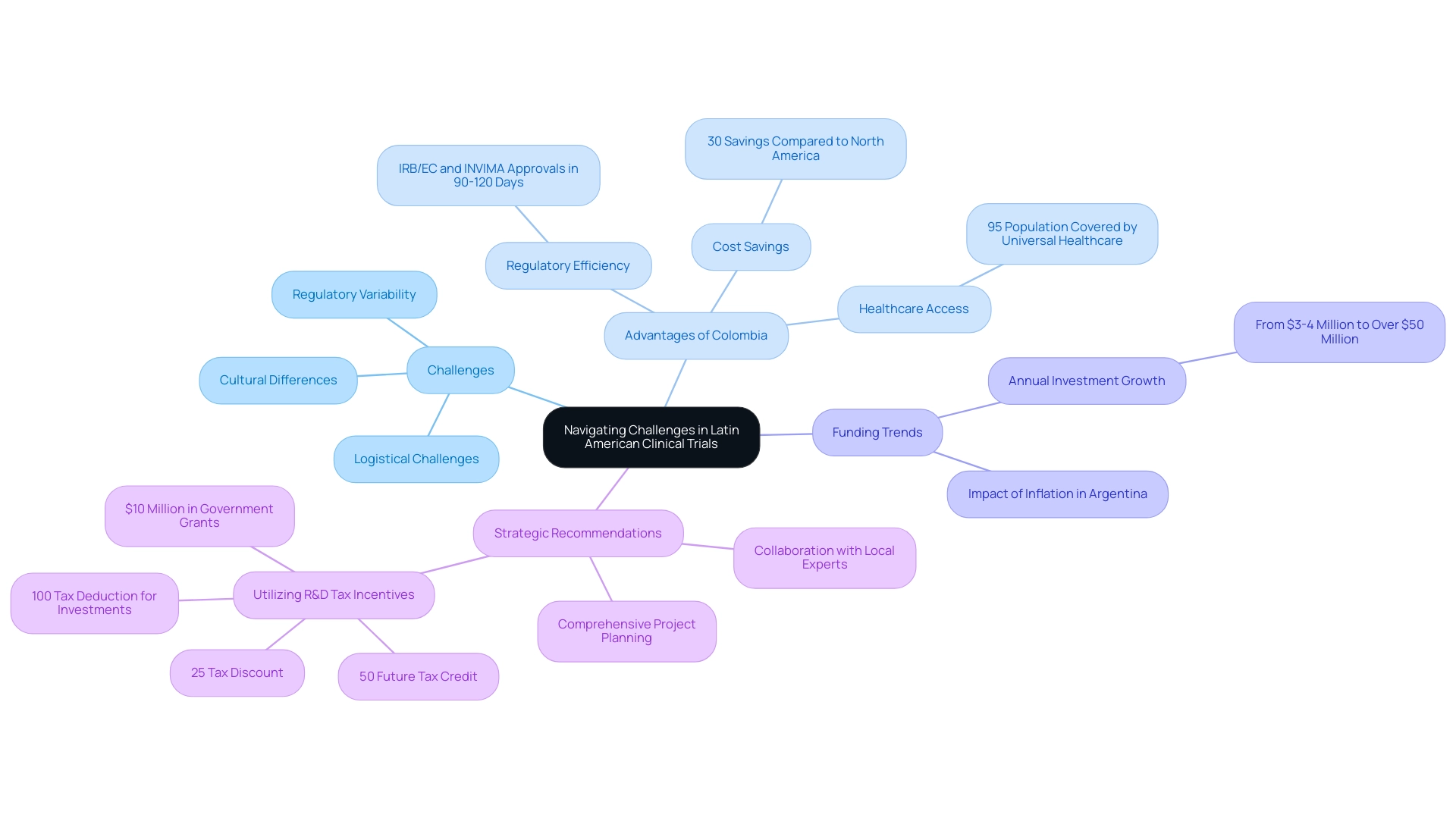
Navigating Challenges in Latin American Clinical Trials
Navigating the complexities of medical studies in South America necessitates a proactive and knowledgeable approach, particularly during the Latin America CRO selection process. Common obstacles in this context include:
- Significant regulatory variability across countries
- Cultural differences that can impact patient engagement
- Logistical challenges related to patient recruitment
Notably, Colombia stands out as a premier location for first-in-human (FIH) studies, offering competitive advantages.
With cost savings exceeding 30% compared to North America and Western Europe, an expedited regulatory process where IRB/EC and INVIMA approvals take merely 90-120 days, and a healthcare system ranked among the best in Latin America, Colombia is increasingly appealing to medical device companies.
Funding in the research sector within the Andean Region has surged from $3-4 million to over $50 million annually, reflecting the growing interest and complexity of conducting studies in this area. Additionally, high inflation in Argentina has influenced this growth, underscoring the economic factors at play. In Colombia, around 95% of the population is covered by universal healthcare, ensuring access to a diverse patient pool for recruitment.
To effectively address these challenges, sponsors should prioritize collaboration with local experts who possess a profound understanding of the regulatory landscape and cultural nuances pertinent to Latin America CRO selection. This regional knowledge is vital, especially as Colombia's initiative for Latin America CRO selection actively seeks to attract more research studies as part of its transformation into a knowledge economy by 2031. As Julio G. Martinez-Clark, CEO of bioaccess®, notes, "The government of Colombia appears to be the only nation in South America with a proactive effort to draw more research studies as part of its strategy to transform into a knowledge economy by 2031."
Moreover, developing a comprehensive project plan that includes contingency strategies is crucial for managing potential delays and ensuring that processes remain on track. The diverse disease landscape in Latin America, characterized by rising incidences of conditions such as cancer, heart disease, and diabetes, presents unique opportunities for specialized patient groups in research studies, particularly in the context of Latin America CRO selection. Specific cancers, such as gastric carcinoma and gallbladder cancer, are prevalent in Chile and Peru, while infectious diseases offer opportunities for specialized patient populations.
This diversity not only enhances recruitment efforts but also facilitates a broader range of studies in the context of Latin America CRO selection.
Additionally, Colombia provides significant R&D tax and financial incentives, including:
- A 100% tax deduction for investments in science, technology, and innovation projects
- A 25% tax discount
- A 50% future tax credit
- Approximately $10 million in government grants
Understanding these incentives can further enhance the appeal of conducting research in Colombia.
In summary, grasping and navigating the regulatory differences in South America is crucial for the success of Latin America CRO selection in conducting medical studies. By leveraging local expertise, utilizing comprehensive research management services—including feasibility studies, compliance reviews, study setup, and project management—and implementing strategic planning, sponsors can overcome common challenges and optimize their research endeavors in this dynamic region.
Best Practices for Selecting a CRO: A Summary
The success of clinical studies hinges on the careful selection of a Latin America CRO. To optimize this essential process, consider the following best practices:
- Define Experiment Objectives and Requirements: Clearly articulating your experiment objectives is paramount. This clarity will guide the selection process and ensure alignment with the CRO's capabilities.
- Evaluate Experience and Expertise: Assess potential CROs based on their experience in the medical technology sector, regulatory knowledge, and proven patient recruitment strategies. A CRO with a strong track record in similar studies can significantly enhance your study's success. For instance, bioaccess®, a leading CRO, exemplifies the importance of Latin America CRO selection, boasting over 15 years of experience in advancing Medtech clinical research and successfully bridging the gap between innovative companies and the potential for conducting clinical studies in the region. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, shared his positive experience with bioaccess® during its initial human study in Colombia, emphasizing their expertise in navigating local challenges.
- Quality Assurance Processes: Ensure that the CRO has robust quality assurance measures in place. This involves compliance with regulatory standards and a commitment to preserving high-quality data throughout the study.
- Foster Open Communication: Establishing a collaborative relationship with your CRO is crucial. Open lines of communication facilitate problem-solving and ensure alignment between both parties throughout the proceedings. As Joel Morse, CEO and co-founder of Curavit Clinical Research, states, "This is a huge step toward improving equitable quality healthcare for all," highlighting the significance of effective partnerships in achieving quality outcomes.
- Engage Local Experts: Navigating the complexities of medical studies in Latin America can be challenging, given the varying healthcare systems and regulatory landscapes across countries. Engaging local experts can provide valuable insights crucial for Latin America CRO selection and help develop contingency plans to address potential obstacles. Dr. John B. Simpson’s research on Avinger’s OCT-guided atherectomy in Cali, Colombia, underscores the importance of collaborating with LATAM CRO experts who understand local market dynamics. Steve Garchow, during his podcast conversation, emphasized the necessity of comprehending local healthcare systems and fostering connections with local stakeholders to enhance market access and success in studies.
By applying these best practices, sponsors can significantly improve the chances of successful research outcomes. Notably, studies indicate that following up with prospects twice can increase response rates by 25%, underscoring the importance of persistent communication in the CRO selection process. This persistent communication not only fosters better partnerships but also contributes to achieving efficient and effective trial results as the landscape of clinical research evolves in 2025.
Conclusion
Selecting the right Contract Research Organization (CRO) in Latin America is paramount for the success of clinical trials, as it directly influences timelines, costs, and the quality of clinical data. This article underscores the necessity of evaluating key criteria such as:
- Experience
- Regulatory knowledge
- Patient recruitment capabilities
- Cultural competence
By opting for a CRO that aligns with these criteria, sponsors can significantly enhance their chances of achieving study objectives and expedite the process of bringing innovative medical technologies to market.
Latin America's diverse patient demographics, cost advantages, and evolving regulatory environments position it as an attractive destination for clinical trials. The region is characterized by lower operational costs and shorter approval timelines, which can greatly enhance the efficiency of clinical research. Furthermore, the commitment of local CROs, such as bioaccess®, to providing tailored solutions and maintaining high standards of regulatory compliance reinforces the potential for successful trial outcomes in this region.
In conclusion, navigating the complexities of clinical trials in Latin America necessitates a strategic approach to CRO selection. By fostering effective communication and collaboration with chosen CROs, sponsors can leverage local expertise and ensure that their clinical trials are not only efficient but also yield high-quality data. As the Medtech sector continues to expand, understanding the unique landscape of Latin America will be essential for driving successful clinical research initiatives in the years to come.
Frequently Asked Questions
Why is selecting a suitable Contract Research Organization (CRO) important for medical studies in Latin America?
Selecting a suitable CRO is crucial because it can streamline processes, enhance patient recruitment, and ensure compliance with regulatory standards, ultimately influencing timelines, costs, and the quality of data generated.
How does the choice of CRO affect research success rates and costs?
A well-chosen CRO can enhance the likelihood of achieving study objectives, which can reduce the average cost of bringing a drug to market, potentially saving over $1 billion.
What services does bioaccess® offer to Medtech companies?
Bioaccess® offers a range of services including feasibility studies, site selection, compliance reviews, pilot studies, first-in-human studies, pivotal studies, and post-market research follow-up studies.
How has bioaccess® demonstrated its effectiveness in clinical trials?
Bioaccess® has achieved significant improvements in recruitment time and retention rates, exemplified by their partnership with GlobalCare Clinical Trials, which resulted in over a 50% reduction in recruitment time and 95% retention rates.
What are the advantages of conducting research studies in Latin America?
Latin America offers a diverse and urbanized population, cost-effectiveness (with expenses potentially up to 50% lower than in North America and Europe), and a favorable regulatory landscape that is becoming more flexible.
What is the typical timeline for regulatory review in Colombia?
The total review time for IRB/EC and MoH (INVIMA) in Colombia typically takes 90-120 days.
How do dropout rates in South America compare to those in the U.S. and EU?
Dropout rates in South America are one-third of those in the U.S. and EU, which enhances the reliability of research outcomes.
What challenges exist for conducting medical studies in Latin America?
Language barriers can complicate logistics, but employing culturally sensitive translation methods can improve the informed consent process and participant engagement.
What financial incentives are available for conducting research in Colombia?
Sponsors can benefit from R&D tax incentives, including a 100% tax deduction, a 25% tax discount, a 50% future tax credit, and approximately $10 million in government grants for investments in science, technology, and innovation projects.
What is the overall significance of Latin America for CRO selection in research studies?
The combination of a diverse population, cost benefits, and a transforming regulatory environment positions Latin America as an ideal site for CRO selection, enhancing research efficiency and supporting economic development and healthcare improvements in the region.

