Overview
The article emphasizes best practices for fostering medtech innovation in Mexico through research, underscoring the critical role of strategic partnerships, regulatory navigation, and innovative methodologies. It asserts that successful medtech development in Mexico hinges on collaboration with local institutions, effective team management, and the adoption of adaptive study designs. These elements are essential for overcoming challenges and enhancing healthcare outcomes, showcasing the necessity of a cohesive approach in this evolving landscape.
Introduction
In a rapidly evolving healthcare landscape, Mexico has emerged as a vibrant hub for medical technology innovation. This transformation is driven by favorable regulatory conditions, a skilled workforce, and increasing investment in healthcare solutions.
Strategically positioned near the United States, Mexico attracts international medtech companies eager to capitalize on its potential for nearshoring and operational optimization. Government initiatives aimed at enhancing research and development further bolster the Mexican medtech sector, which is not only transforming the local economy but also gaining recognition on the global stage.
As the industry prepares for significant advancements in areas such as artificial intelligence and clinical trial methodologies, it is crucial to explore how these developments are reshaping the future of healthcare delivery and patient outcomes in the region.
The Landscape of Medtech Innovation in Mexico
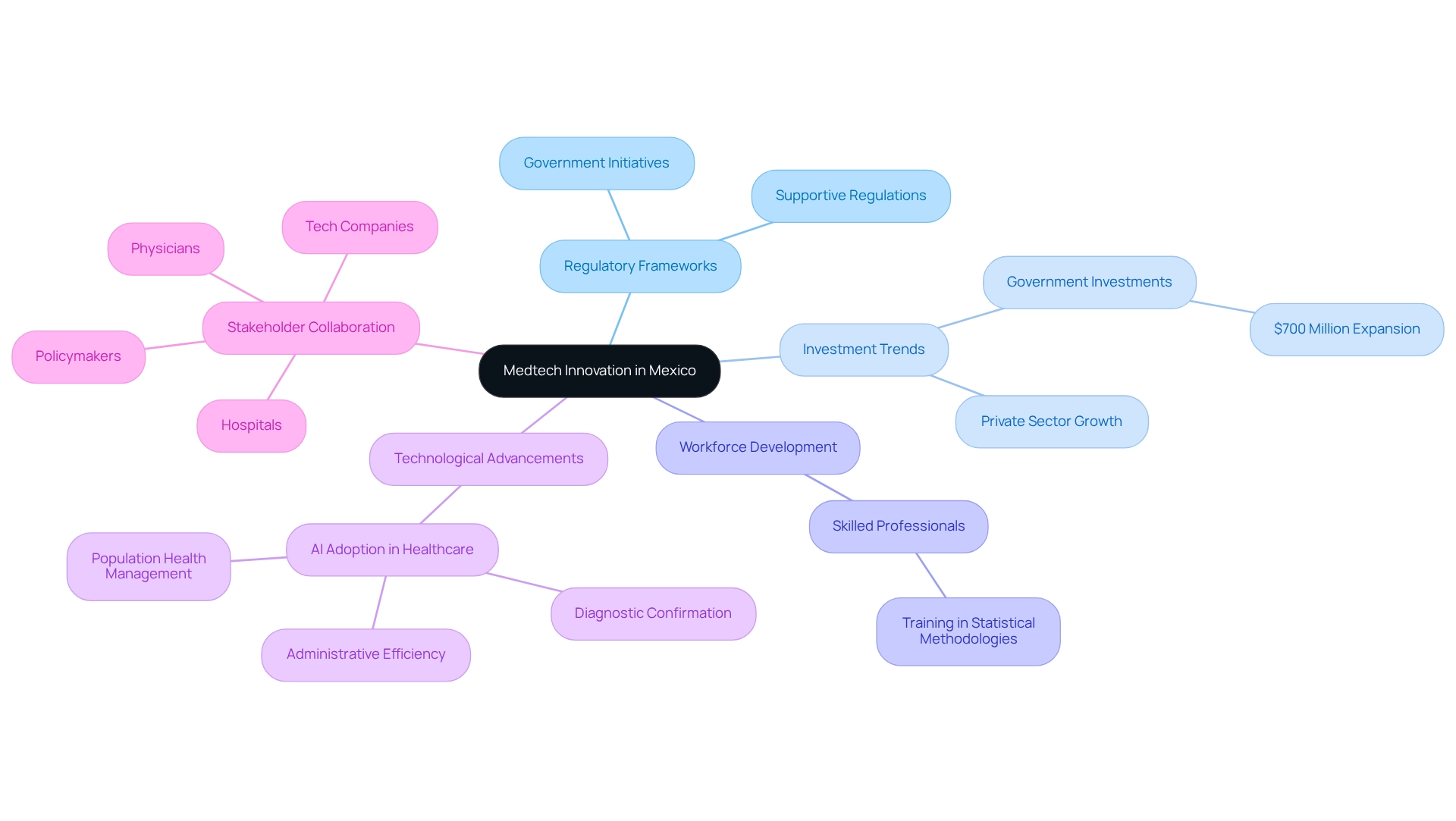
Mexico has established itself as a dynamic center for medtech innovation through its research, bolstered by a confluence of regulatory frameworks, a highly skilled workforce, and a notable increase in investments in healthcare technologies. The country's strategic proximity to the United States enhances its appeal for nearshoring, making it a prime location for international medtech enterprises seeking to optimize operations. Recent government initiatives aimed at enhancing development within the healthcare sector have further fostered an environment conducive to innovation.
In 2025, Mexico is experiencing a remarkable uptick in the creation of medical devices, encompassing everything from cutting-edge diagnostic tools to sophisticated surgical instruments. This growth is not only transforming the local economy—evidenced by significant job creation and improvements in healthcare infrastructure—but also positioning Mexico as a pivotal player in the global medtech arena. Clinical studies conducted by bioaccess in Mexico significantly impact local economies by creating jobs, enhancing healthcare infrastructure, and fostering international collaboration, which solidifies the country's role in medtech innovation and contributes to its international recognition.
Investment trends reflect this momentum, with substantial financial commitments, such as a $700 million expansion in one of Mexico's largest hospital networks, resulting in the establishment of 15 new facilities. Such developments underscore the increasing confidence in Mexico's medtech landscape, further supported by expert opinions recognizing the country's potential as a medtech hub. As Howard Barkan, an associated researcher and consulting statistician, highlights, 'Statistics in medical studies: Significant factors underline the necessity of training in statistical methodologies.' This emphasizes the need for skilled professionals in the medtech sector to drive innovation.
Additionally, experts predict that by 2025, medtech innovation could significantly advance AI adoption in healthcare, with potential applications in diagnostic confirmation, administrative efficiency, and population health management. Realizing these benefits will depend on collective efforts from physicians, hospitals, policymakers, and tech companies to provide necessary training, develop infrastructure, and enact supportive regulations. As the landscape evolves, the collaborative efforts of stakeholders—including physicians, hospitals, policymakers, and technology firms—will be crucial in harnessing the full benefits of these advancements, ultimately enhancing patient outcomes and modernizing healthcare delivery.
The accompanying visual representation, a stylized icon depicting a globe or network in teal, symbolizes the connectivity and global reach of these initiatives.
Challenges in Clinical Research for Medtech Companies
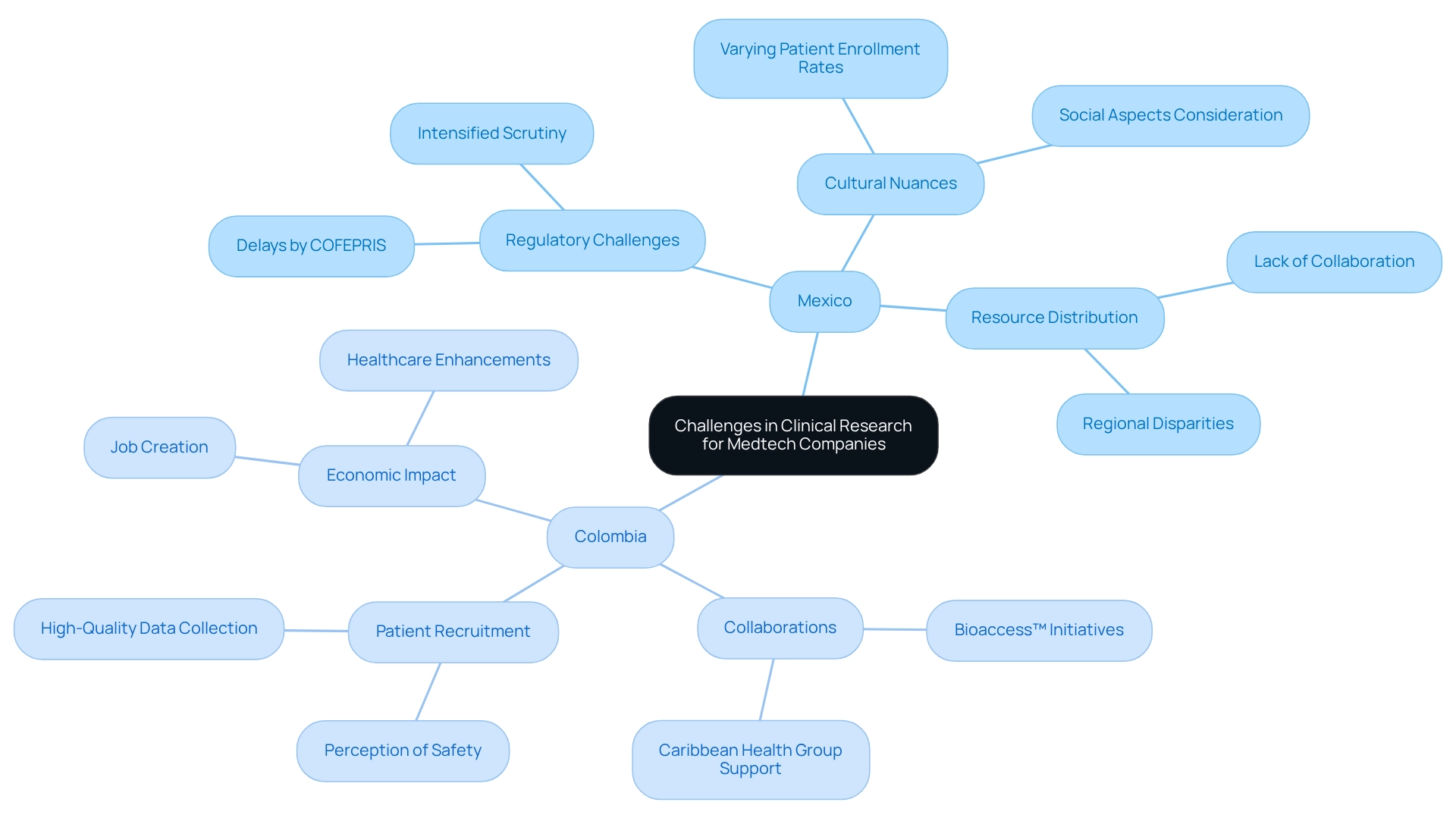
The Mexican medtech industry, while promising, confronts significant obstacles in medical studies. Regulatory challenges, particularly those imposed by COFEPRIS, the Mexican regulatory authority, often result in delays in approval processes. In 2025, the scrutiny of approval timelines for medical studies has intensified, with companies facing extended waiting periods that can disrupt their development schedules.
Additionally, cultural nuances and varying patient enrollment rates across different regions complicate the execution of research studies. Companies frequently face the dual challenge of ensuring compliance with local regulations while maintaining the integrity of their research. The absence of established networks for collaboration can hinder the sharing of resources and knowledge, which is essential for startups striving to navigate this competitive environment. A case study focusing on infrastructure and resource distribution in medical studies highlights that, despite Mexico's robust healthcare system, regional disparities in study capacity necessitate careful evaluation and resource allocation to effectively conduct medical experiments.
As reported in the news, addressing these challenges requires a strategic approach that takes into account both the regulatory landscape and the socio-cultural dynamics at play, ultimately facilitating medtech innovation through Mexican research in the region.
In contrast, Colombia has emerged as a promising destination for research trials, bolstered by initiatives from bioaccess™ and Caribbean Health Group. Their collaboration aims to position Barranquilla as a leading center for health studies in Latin America, supported by Colombia's Minister of Health. This partnership not only boosts the local economy through job creation and healthcare enhancements but also promotes international collaboration, propelling global health innovation.
Julio Martinez-Clark underscores that Colombia’s reputation has transformed into one of knowledge, where companies can feel secure and at ease, thereby facilitating patient recruitment for high-quality data. This highlights the crucial role of collaboration and the perception of safety in enhancing patient recruitment efforts.
Building Strategic Partnerships for Enhanced Research Capabilities
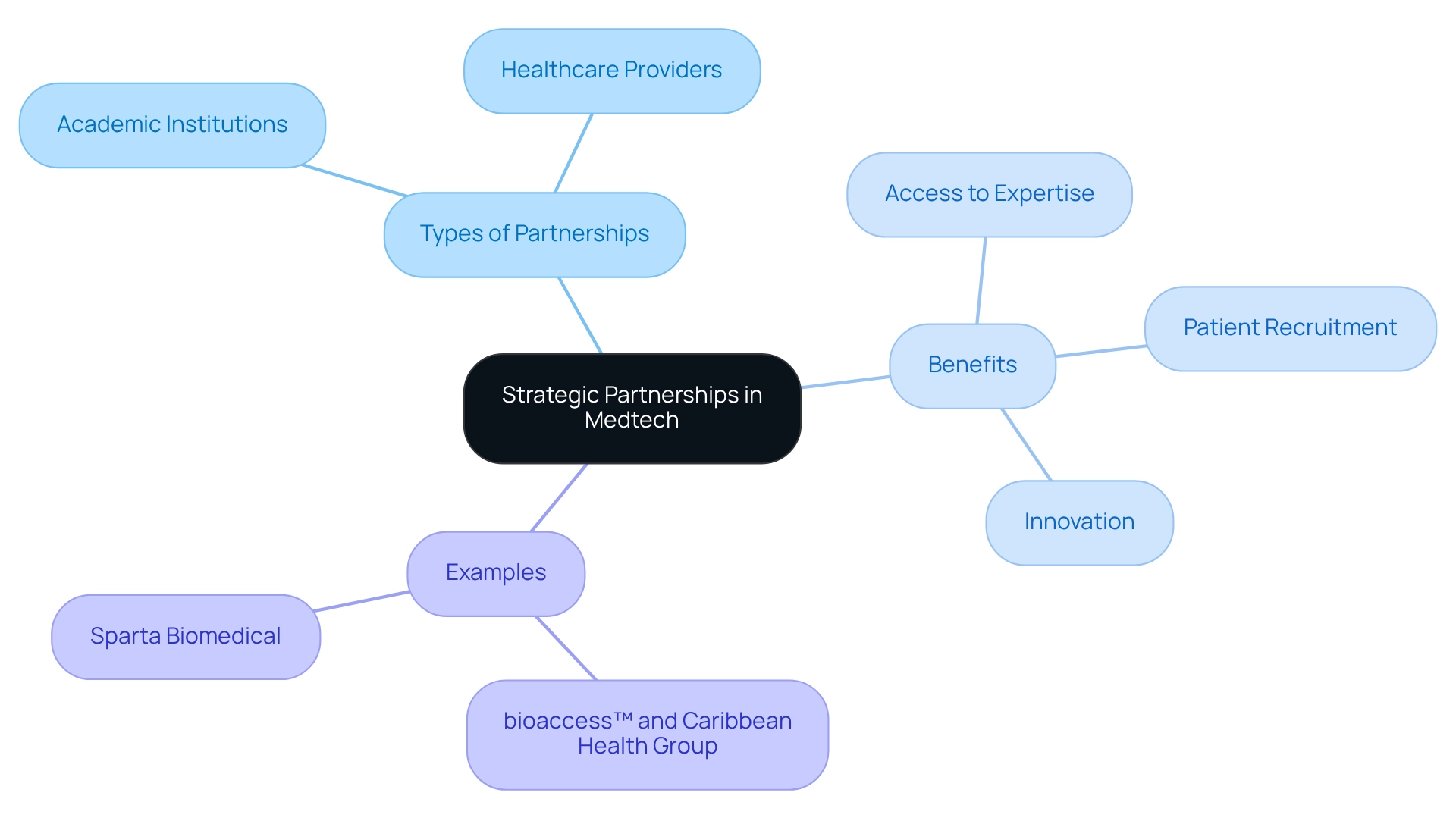
To successfully navigate the intricacies of medical studies, Medtech companies must prioritize forming strategic alliances with local institutions, universities, and healthcare providers. Such collaborations significantly enhance investigative capabilities by providing access to specialized expertise, diverse patient populations, and advanced technologies. Partnerships with academic institutions facilitate the recruitment of qualified researchers and offer valuable insights into the latest scientific advancements, pivotal for innovation.
Collaborating with local healthcare providers further strengthens these efforts by improving patient recruitment and retention rates, crucial for the success of research trials. In 2025, the landscape of Medtech investigation in Mexico has witnessed a significant rise in partnerships, with statistics indicating that over 60% of Medtech firms are collaborating with academic institutions to enhance their trials. This trend underscores the importance of strategic partnerships in driving innovation and accelerating the development of new medical technologies.
A prime illustration of this is bioaccess™, a prominent contract study organization in Latin America, effectively connecting innovative Medtech companies with opportunities for conducting trials in the region. Their partnership with Caribbean Health Group, revealed on March 29, 2019, aims to establish Barranquilla as a leading destination for medical research in Latin America, an initiative supported by Colombia's Minister of Health. This collaboration not only enhances the area's investigative capabilities but also attracts more clinical study initiatives, fostering a robust environment for medical advancement.
Moreover, successful partnerships between Medtech firms and academic institutions have been documented in various case studies, demonstrating how these alliances can lead to groundbreaking progress in medical device development. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, shared his positive experience with bioaccess® during its initial human study in Colombia, underscoring the effectiveness of such partnerships. As Elon Musk aptly stated, "Persistence is very important. You should not give up unless you are forced to give up." By nurturing these relationships, companies can establish a strong support network that not only drives innovation but also ensures that new medical technologies reach the market more swiftly and effectively.
Best Practices for Recruiting and Managing Research Teams
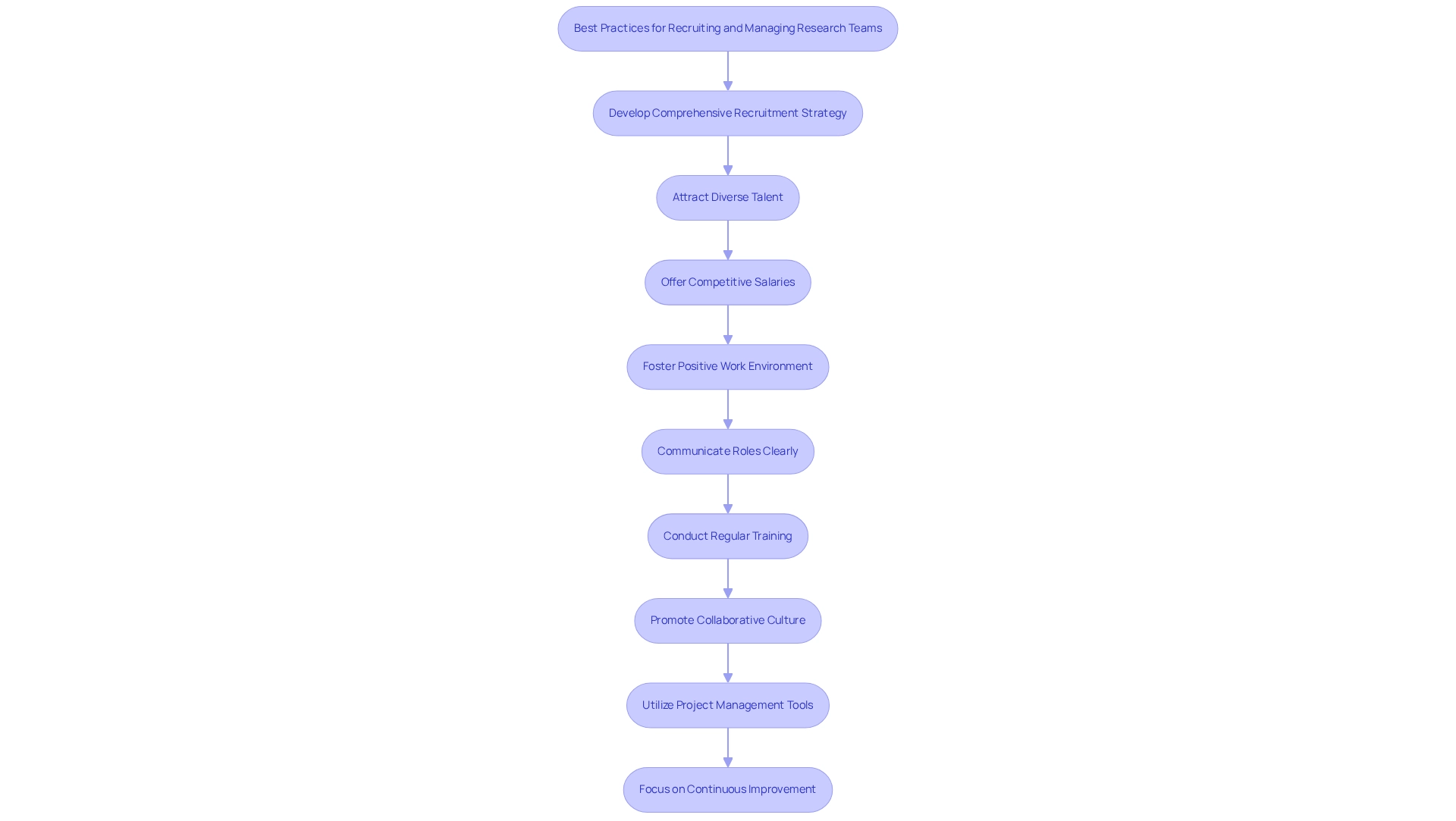
Attracting and overseeing a talented study group is essential for the success of medical technology projects in the ever-changing environment of Latin America. Companies should adopt a comprehensive recruitment strategy that prioritizes attracting diverse talent with the necessary expertise. This can be achieved by offering competitive salaries, opportunities for professional development, and fostering a positive work environment that values inclusivity.
Data suggest that workforce diversity in medical study teams in Mexico is increasing, which can result in more innovative solutions and enhanced trial outcomes.
Once a team is formed, applying effective management practices is crucial. Clear communication of roles and responsibilities, regular training sessions, and the promotion of a collaborative culture are vital components. Moreover, utilizing project management tools can simplify workflows and improve team productivity, facilitating more effective navigation of the complexities inherent in research.
In 2025, as the research landscape becomes more competitive, it is crucial for Medtech companies to concentrate on these best practices. Bree Burks, Vice President of Site Strategy at Veeva, observes that as sponsors rethink their site engagement methods, they will focus on uniform site technology and standardization among sponsors for all research. Moreover, the proficiency of bioaccess® in overseeing research studies, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, establishes them as a premier Contract Research Organization in Latin America.
Bioaccess® provides extensive services including feasibility assessments, site selection, compliance evaluations, project organization, import permits, project management, and reporting, guaranteeing a detailed approach to research initiatives.
The influence of research efforts on local economies is significant, as they aid in job creation, economic development, and enhancements in healthcare. The case study titled '7 Essential Clinical Trial Design Strategies' outlines important strategies for trial design that are essential for advancing medical investigations, particularly in the context of first-in-human studies. Furthermore, Max Baumann from Treehill Partners cautions about essential business model difficulties in biotech as the medical market becomes more congested.
By emphasizing these strategies and utilizing local knowledge, organizations can create adaptable teams able to tackle the challenges of Medtech innovation through Mexican research and achieve successful research outcomes. For individuals aiming to navigate the intricacies of medical studies in Latin America, collaborating with bioaccess® can offer the essential knowledge and assistance to attain success.
Implementing Innovative Methodologies in Clinical Trials
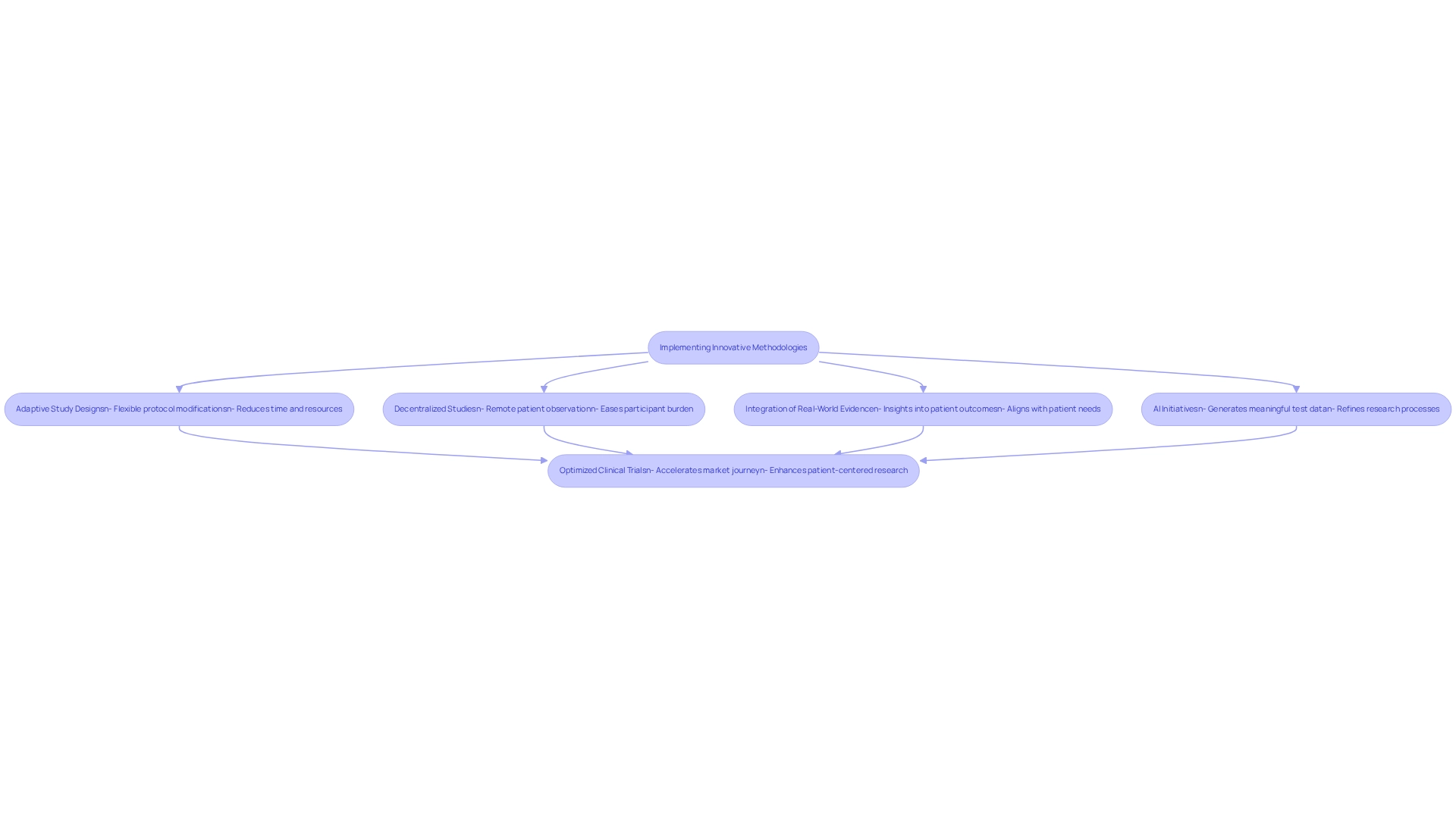
To enhance the effectiveness of medical research, medtech firms must prioritize innovation through Mexican research by embracing cutting-edge approaches such as adaptive study designs, decentralized studies, and the integration of real-world evidence. Adaptive study designs are particularly advantageous as they allow for modifications to the protocol based on interim outcomes, fostering a more flexible and responsive investigative environment. This adaptability can significantly reduce the time and resources necessary to achieve definitive outcomes.
Decentralized studies further revolutionize the medical research landscape by leveraging technology to facilitate remote patient observation and data collection. This method not only eases the burden on participants but also bolsters recruitment efforts, making it simpler to gather diverse patient populations. For instance, the Dominican Republic is witnessing an increase in medical device studies, driven by its growing population of over 11 million and heightened interest from medtech firms.
Moreover, incorporating real-world evidence into research can yield invaluable insights into patient outcomes and treatment effectiveness. This evidence-based approach supports the development of medical devices that are not only innovative but also aligned with actual patient needs and experiences. Recent advancements within Mexico’s healthcare system, including a $700 million investment resulting in 15 new hospital facilities, illustrate that the environment for conducting medical studies is strengthening, thereby supporting medtech innovation through Mexican research.
This investment presents significant opportunities for bioaccess® to facilitate these studies, aiding in the expedited advancement of medical devices, which reflects medtech innovation through Mexican research, due to its expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies.
Furthermore, innovative initiatives, such as the partnership between bioaccess® and Caribbean Health Group, supported by Colombia's Minister of Health, aim to position Barranquilla as a premier research hub in Latin America. This collaboration is expected to enhance ambulatory services for research in Colombia, achieving over a 50% reduction in recruitment time and 95% retention rates.
Additionally, projects like the AI initiative led by Ibrahim Kamstrup-Akkaoui highlight the potential for generating meaningful test data that can refine research processes. The algorithm developed analyzes previous studies, learns from real data collected, and utilizes this information to produce valuable insights for new inquiries.
By implementing these techniques, medtech firms can optimize their trials, ultimately accelerating the journey to market for new medical devices. The adoption rates of these innovative methodologies are projected to rise significantly in 2025, reflecting a broader trend towards more efficient and patient-centered clinical research. Moreover, case examples such as Enliven Health's Patient Engagement Network demonstrate how the integration of advanced data insights and digital engagement technologies can enhance patient health outcomes, foster brand loyalty, and stimulate revenue growth for healthcare providers.
The Role of Pilot and Early-Feasibility Studies in Innovation
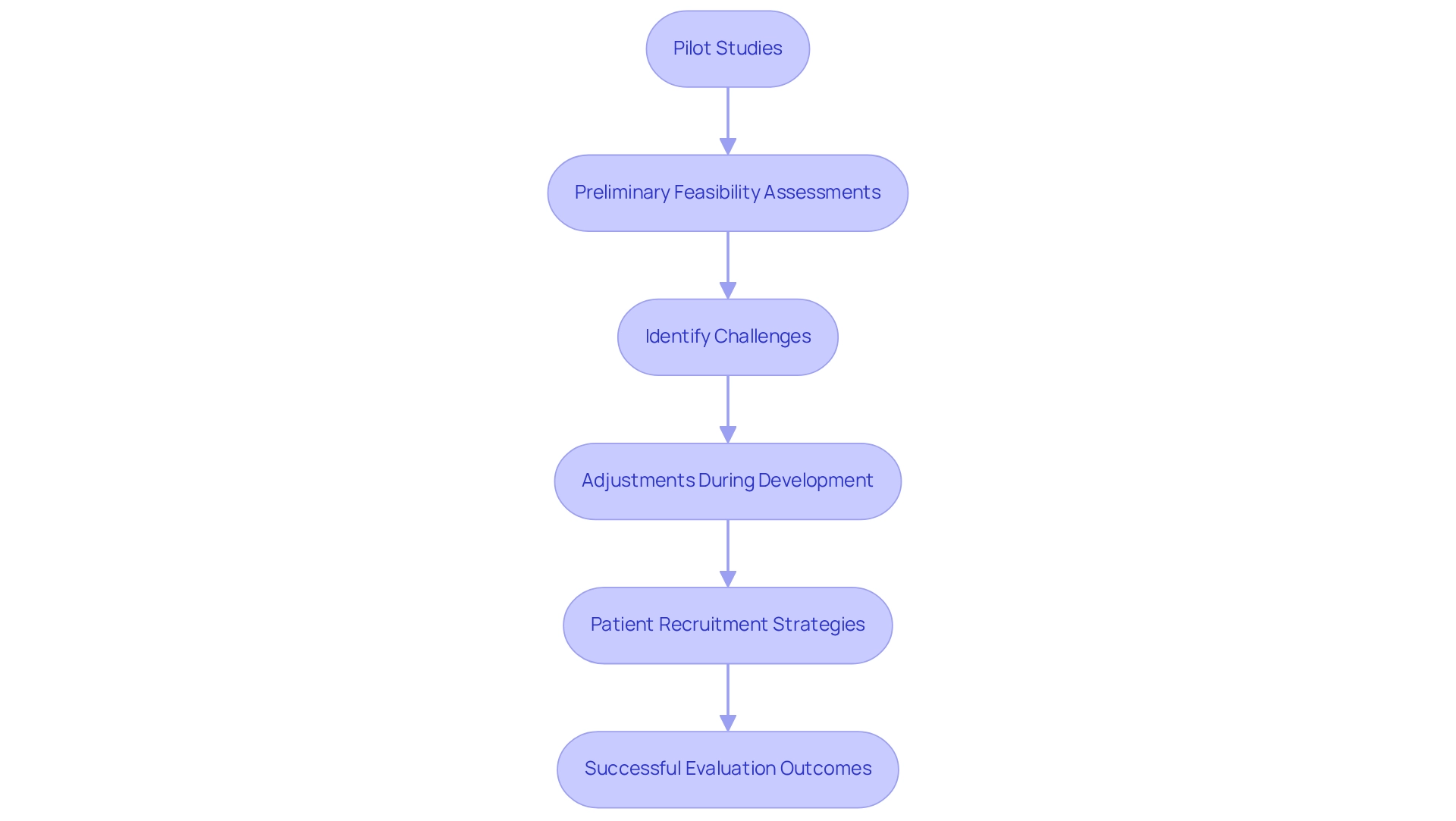
Pilot and preliminary feasibility assessments are essential components of the medtech innovation process, enabling companies to evaluate their concepts in real-world environments prior to extensive testing. These initial investigations are critical in identifying potential challenges related to device design, usability, and patient acceptance, allowing for timely adjustments during development. For instance, a preliminary investigation can yield valuable insights into effective patient recruitment strategies and aid in refining the medical endpoints to be assessed in subsequent experiments.
By underscoring the importance of these preliminary investigations, medtech firms can significantly mitigate risks and enhance their prospects for success in later evaluations.
Looking ahead to 2025, the anticipated success rates of early feasibility assessments in medical experiments are expected to rise, reflecting an increasing recognition of their significance in the development process. This is underscored by recent case studies, such as ReGelTec's Early Feasibility Assessment in Barranquilla, Colombia, where eleven patients were successfully treated with HYDRAFIL™. This research illustrates how bioaccess® provides comprehensive management services for trials, including:
- feasibility assessments
- site selection
- First-In-Human assessments
- Pivotal assessments
- Post-Market Follow-Up assessments
These services are vital for navigating the complexities of evaluations in Latin America.
Moreover, expert insights further highlight the necessity of these investigations; as Porta noted, a pilot examination serves as "a small-scale assessment of the methods and procedures to be utilized on a broader scale," emphasizing that thorough preparation and proactive engagement with regulatory agencies are crucial for successful applications. With over 20 years of experience in managing trials, bioaccess® is well-equipped to support medtech companies in optimizing their development processes. By prioritizing pilot and early feasibility studies, medtech companies can bolster their chances of success and drive innovation through Mexican research, which not only enhances patient outcomes but also fosters economic growth in local communities through job creation and healthcare improvement.
Post-Market Clinical Follow-Up: Ensuring Long-Term Success
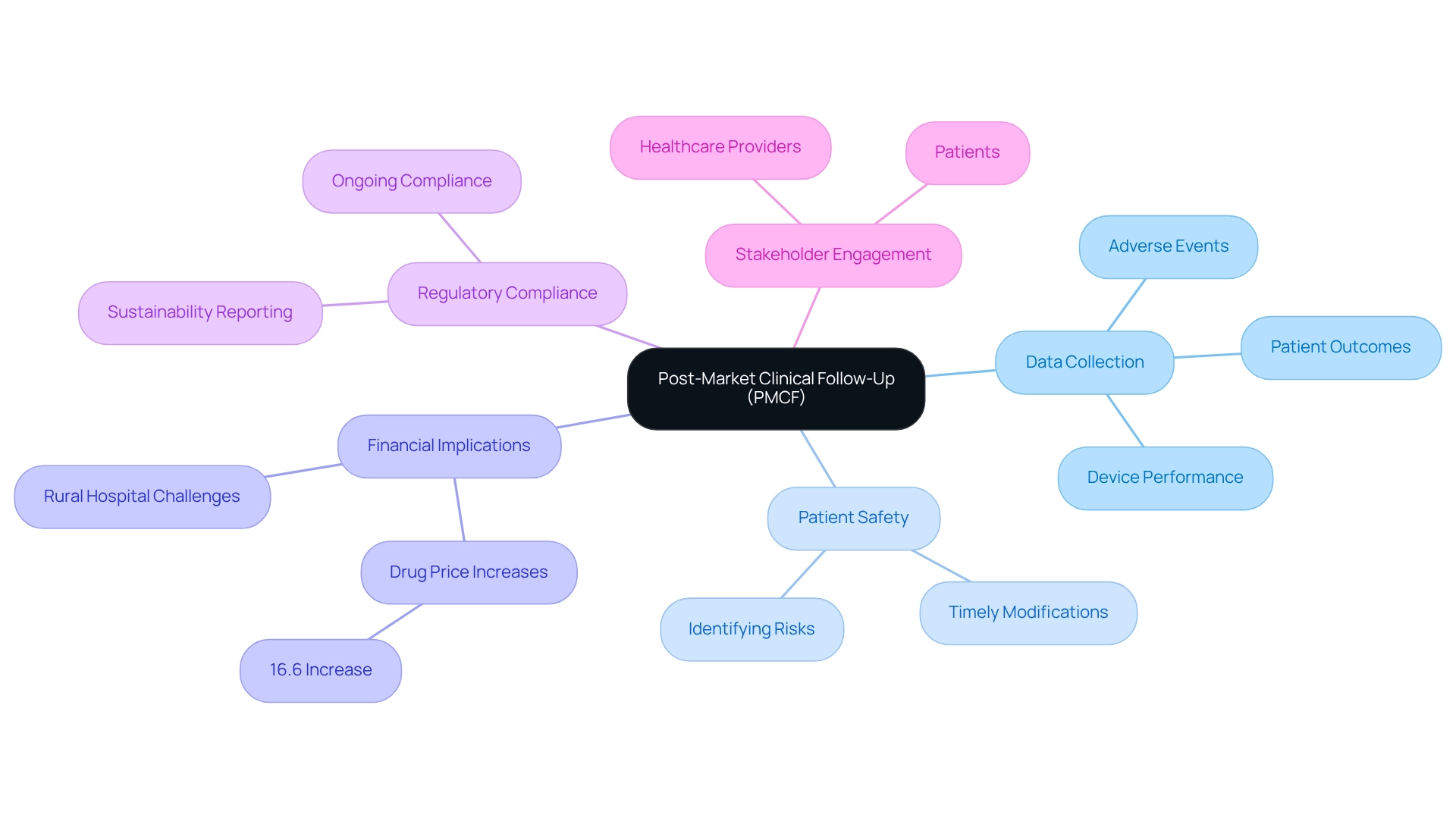
Post-market medical follow-up (PMCF) is vital in the medical device lifecycle, ensuring that products meet safety and efficacy standards consistently after market introduction. At bioaccess®, we recognize that achieving this goal requires companies to develop comprehensive PMCF plans that incorporate systematic data collection on device performance, adverse events, and patient outcomes. Engaging healthcare providers and patients is essential for gathering real-world data, which is invaluable for evaluating long-term device performance and safety. As Darshan Gosalia, Research Manager at Deloitte Center for Health Solutions, emphasizes, the integration of real-world evidence is crucial for medtech innovation through Mexican research, particularly in understanding the long-term impacts of devices on patient health.
Furthermore, PMCF activities are instrumental in identifying previously unknown risks, enabling timely product modifications that enhance patient safety. By prioritizing post-market follow-up, bioaccess® not only improves patient safety but also ensures ongoing regulatory compliance, ultimately supporting the long-term success of medical devices in Latin America.
In addition to PMCF, bioaccess® specializes in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Pilot Studies, leveraging over 20 years of Medtech expertise to navigate trial complexities. The ASPE report highlights a significant financial aspect, revealing up to a 16.6% increase in the prices of drugs in shortage, underscoring the financial implications of PMCF. Ensuring device affordability and access is critical, especially as hospitals, particularly in rural areas, confront substantial financial challenges in maintaining essential services.
Moreover, the European Union's Corporate Sustainability Reporting Directive underscores the necessity for compliance and sustainability in the industry, aligning PMCF with broader trends increasingly relevant in 2025. Adopting best practices for PMCF will be essential for sustaining Medtech innovation through Mexican research while fulfilling the demands of regulatory bodies and healthcare providers.
Continuous Improvement in Clinical Research Practices
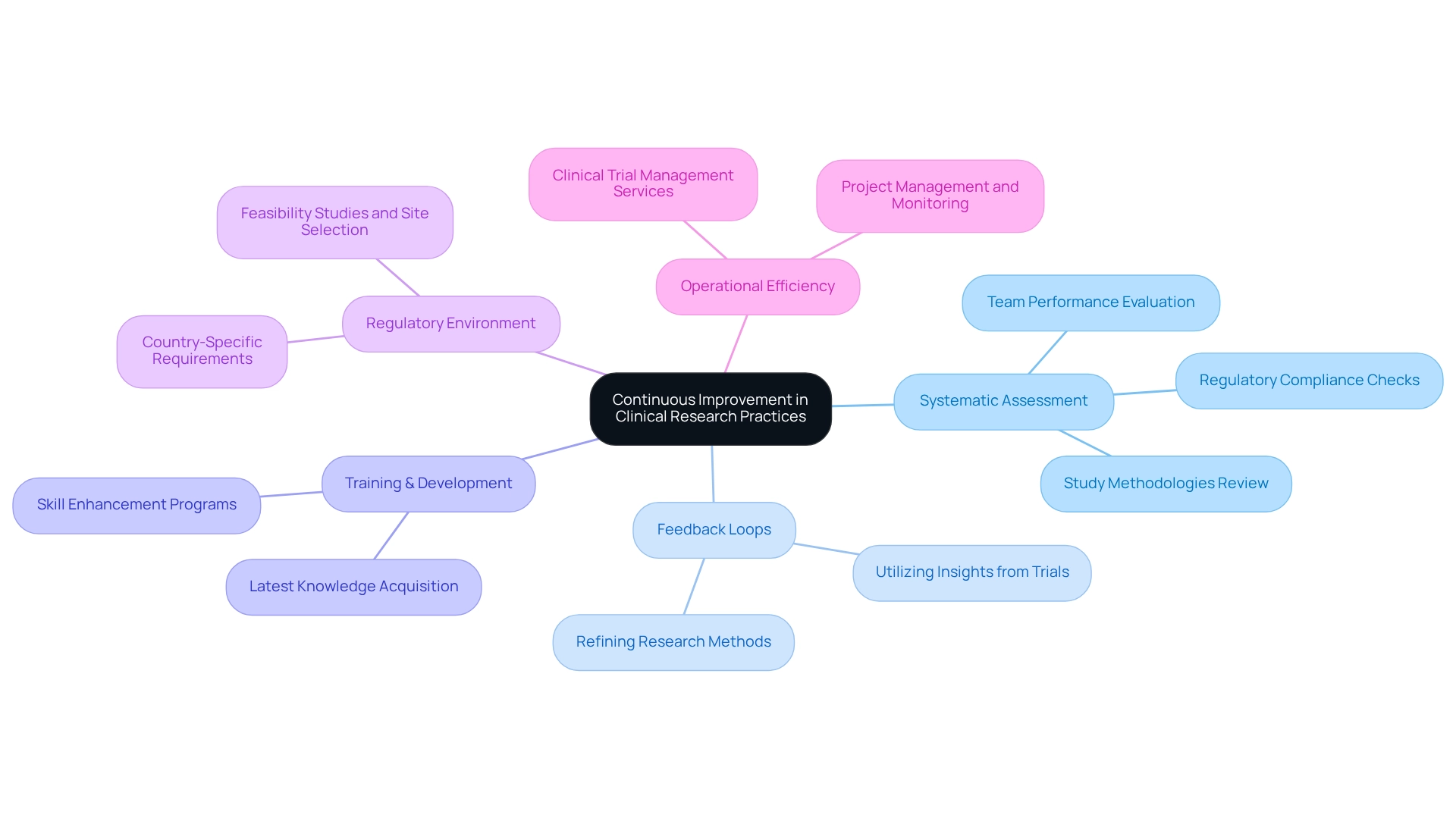
In the fast-paced medtech environment, promoting a culture of ongoing enhancement in clinical practices is crucial for companies like bioaccess striving to retain a competitive advantage. This approach necessitates a systematic assessment of study methodologies, team performance, and compliance with regulatory standards to identify opportunities for improvement. One effective strategy is the implementation of feedback loops, where insights gained from completed trials are utilized to refine and inform future research. This iterative process not only improves investigation strategies but also leads to more robust results.
Investing in the training and development of investigation teams is equally vital. By equipping teams with the latest knowledge and skills, organizations can ensure alignment with evolving industry trends and best practices. For instance, a recent study on bidirectional communication between primary care and behavioral health demonstrated that improved information exchange significantly enhances treatment outcomes. This principle can be applied in medtech studies, where efficient communication and teamwork can result in enhanced patient care and more successful trials.
As we approach 2025, the emphasis on ongoing enhancement practices in medical studies will only intensify. Companies that prioritize these strategies will not only enhance their research capabilities but also contribute to Medtech innovation through Mexican research in the development of medical devices. The CDC Clinical Practice Guideline for Prescribing Opioids for Pain underscores the significance of incorporating evidence-based practices into workflows, aligning with the theme of ongoing enhancement.
Expert opinions indicate that adopting these methodologies is essential for navigating the increasingly crowded healthcare end-markets, as highlighted by Max Baumann, who pointed out that biotech encounters fundamental business model challenges. Additionally, the 2025 MIPS IA_PM_5 activity promotes teamwork with essential partners to apply evidence-based practices, reinforcing the ongoing trend of continuous enhancement in medical research.
In the context of Latin America, understanding the distinct regulatory environment and leveraging local knowledge is vital. Firms such as bioaccess must navigate various country-specific requirements, including feasibility studies, site selection, compliance reviews, and setup for testing, to ensure successful market access. By establishing comprehensive clinical trial management services that encompass import permits, project management, and monitoring, bioaccess can enhance operational efficiency and contribute to local economies through job creation and improved healthcare outcomes.
This proactive approach not only fosters international collaboration but also positions bioaccess to capitalize on the growing demand for Medtech innovation through Mexican research in the region.
Conclusion
Mexico's emergence as a leading hub for medical technology innovation is underscored by a combination of favorable regulatory conditions, a skilled workforce, and increasing investments. The strategic positioning of the country, particularly in relation to the United States, enhances its attractiveness for international medtech companies looking to optimize their operations. The significant growth in medical device creation and the establishment of new healthcare facilities illustrate how the sector is transforming the local economy and gaining global recognition.
However, the journey is not without challenges. Regulatory hurdles and cultural nuances complicate clinical research, necessitating a strategic approach to navigate these complexities. Establishing partnerships with local institutions is essential for enhancing research capabilities and successfully conducting clinical trials. The rise in collaborative efforts among medtech companies, universities, and healthcare providers signals a promising trend toward driving innovation and improving patient outcomes.
As the industry looks ahead to 2025, the integration of innovative methodologies such as adaptive trial designs and decentralized trials will be crucial in streamlining clinical research processes. Additionally, the emphasis on post-market clinical follow-up ensures that medical devices continue to meet safety and efficacy standards, fostering long-term success.
In summary, Mexico's medtech sector is poised for significant advancements, driven by a collaborative spirit and a commitment to continuous improvement. By leveraging local expertise and embracing innovative practices, stakeholders can navigate the evolving landscape effectively, ultimately enhancing healthcare delivery and patient outcomes in the region. The future of medical technology in Mexico not only holds promise for local economies but also positions the nation as a key player on the global stage.
Frequently Asked Questions
What factors contribute to Mexico's position as a center for medtech innovation?
Mexico's medtech innovation is supported by a combination of regulatory frameworks, a highly skilled workforce, increased investments in healthcare technologies, and government initiatives aimed at enhancing the healthcare sector.
How is Mexico's proximity to the United States beneficial for medtech enterprises?
Mexico's strategic proximity to the United States makes it an attractive location for nearshoring, allowing international medtech companies to optimize their operations effectively.
What significant developments are expected in Mexico's medical device industry by 2025?
By 2025, Mexico is expected to see a significant increase in the production of medical devices, including advanced diagnostic tools and surgical instruments, contributing to job creation and improvements in healthcare infrastructure.
How do clinical studies in Mexico impact the local economy?
Clinical studies conducted in Mexico create jobs, enhance healthcare infrastructure, and foster international collaboration, solidifying Mexico's role in medtech innovation and increasing its global recognition.
What recent investment trends are occurring in Mexico's healthcare sector?
Recent investment trends include substantial financial commitments, such as a $700 million expansion in one of Mexico's largest hospital networks, leading to the establishment of 15 new facilities, which reflects growing confidence in Mexico's medtech landscape.
What challenges does the Mexican medtech industry face regarding medical studies?
The Mexican medtech industry faces challenges such as regulatory hurdles from COFEPRIS, extended approval timelines, cultural nuances affecting patient enrollment, and a lack of established networks for collaboration.
How does the regulatory environment affect medical studies in Mexico?
The regulatory environment, particularly scrutiny from COFEPRIS, can lead to delays in approval processes for medical studies, disrupting development schedules for companies.
What is the significance of collaboration among stakeholders in advancing medtech innovation in Mexico?
Collaboration among physicians, hospitals, policymakers, and technology firms is crucial for harnessing the benefits of medtech advancements, enhancing patient outcomes, and modernizing healthcare delivery.
How does Colombia compare to Mexico in terms of medtech research opportunities?
Colombia is emerging as a promising destination for research trials, supported by initiatives from organizations like bioaccess™ and Caribbean Health Group, which aim to enhance patient recruitment and promote international collaboration.
What role does patient perception play in the success of medtech research in Colombia?
Patient perception of safety and the reputation of Colombia as a knowledge hub facilitate patient recruitment for high-quality data, which is essential for successful medical studies.




