Overview
The article primarily focuses on best practices for designing post-market trials in Brazil, underscoring the critical role these studies play in ensuring the safety and efficacy of medical devices. It delineates essential strategies, including:
- Stakeholder engagement
- Adherence to regulatory requirements
- Utilization of real-world evidence
Together, these elements significantly enhance the credibility and effectiveness of post-market evaluations within Brazil's rapidly evolving medical device landscape.
Introduction
In the dynamic realm of medical devices, the significance of post-market trials is paramount. These trials function as a vital mechanism for ensuring the ongoing safety and effectiveness of devices after they enter the market, addressing potential issues that may not have surfaced during pre-market evaluations.
As Brazil's medical device landscape continues to flourish, the role of post-market trials becomes increasingly critical for both manufacturers and regulatory authorities. This article explores the complexities of post-market trials in Brazil, examining their objectives, regulatory frameworks, and the challenges encountered in designing effective studies.
It underscores the collaborative approaches essential for engaging stakeholders and highlights best practices that can enhance trial outcomes. With innovations on the horizon, grasping the evolving landscape of post-market trials is essential for cultivating public trust and advancing healthcare solutions.
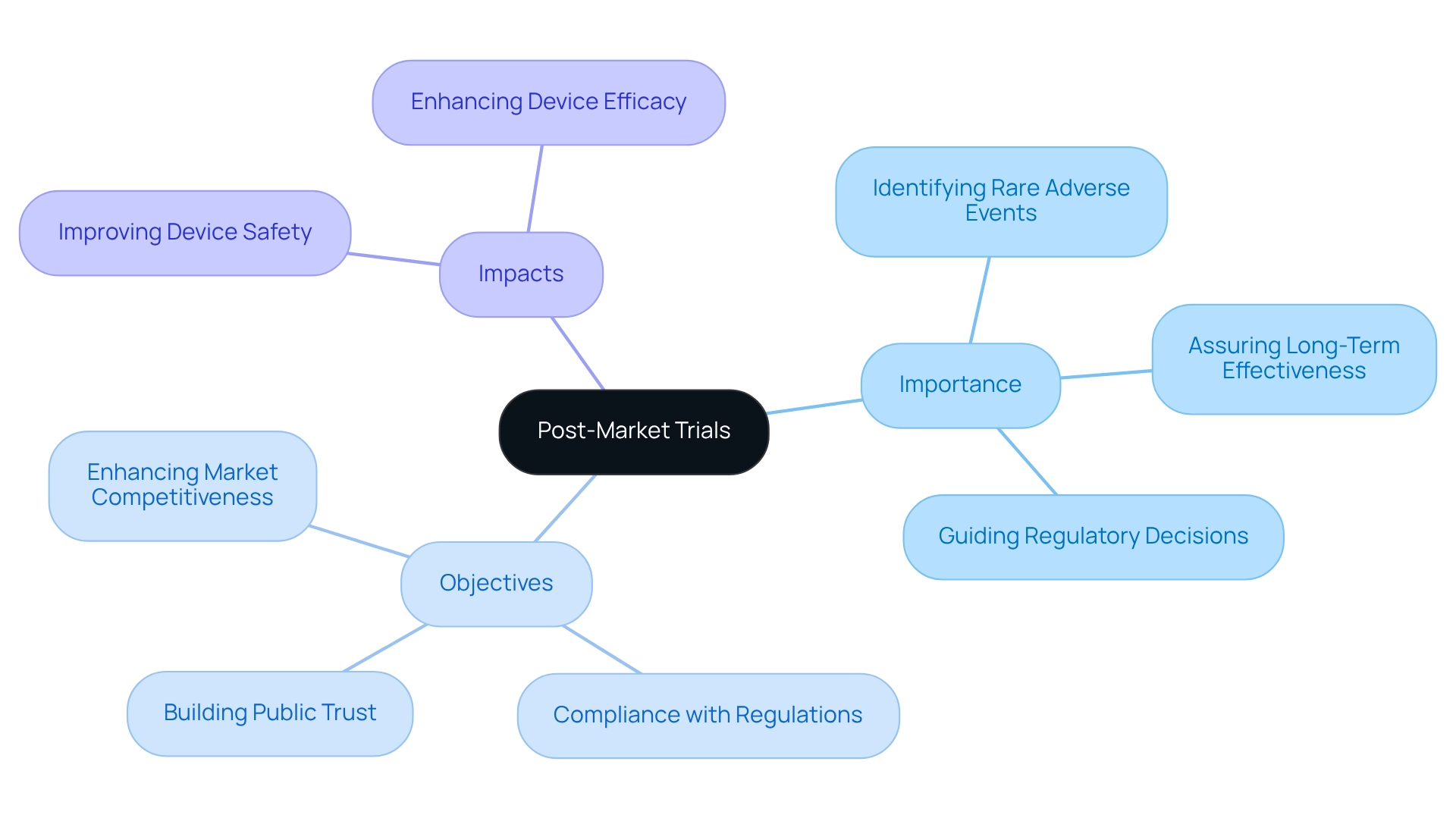
Understanding Post-Market Trials: Importance and Objectives
Post-market studies are vital for the ongoing evaluation of the safety and efficacy of medical devices once they enter the market. These studies serve several essential purposes:
- They identify rare adverse events that may not have been apparent during pre-market evaluations.
- They assess the long-term effectiveness of devices.
- They generate insights that can guide future regulatory decisions.
In Brazil, where the medical device market is rapidly expanding, the importance of post-market trial design cannot be overstated for both manufacturers and regulatory authorities.
These studies not only ensure compliance with local regulations but also foster public confidence in medical technologies. The necessity for thorough oversight underscores the significance of monitoring after market entry, enhancing market competitiveness and building trust among healthcare providers. For instance, a recent case study on the development of a 59-item survey tool demonstrated that manufacturers engaged in after-market research could significantly improve their practices. This tool was refined through literature reviews and expert consultations, ultimately facilitating the collection of valuable data on manufacturers' involvement in post-market studies.
This directly aligns with the objectives of the post-market trial design in Brazil, highlighting the need for continuous improvement in clinical practices. As Brazil's medical device landscape continues to evolve, particularly in 2025, the relevance of post-market trial design becomes increasingly pronounced. These evaluations are crucial for identifying adverse events, with data indicating that a substantial proportion of adverse occurrences arise post-launch, emphasizing the need for ongoing vigilance. Regulatory reporting requirements outlined in 21 CFR Part 806 for device corrections and removals further stress the compliance obligations manufacturers must adhere to, reinforcing the significance of these evaluations.
Moreover, real-world examples illustrate how after-market trials have led to improvements in device safety and effectiveness, underscoring the critical role of these studies within the regulatory framework. As the medical device industry advances, the commitment to after-market trials will be essential in ensuring that devices not only meet initial safety and efficacy standards but also sustain effective performance in real-world settings. bioaccess®'s expertise in managing follow-up studies post-market release, along with Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, and Pivotal Studies, ensures that manufacturers can navigate these challenges effectively, preserving the integrity and reliability of medical technologies. With over 20 years of experience in Medtech, bioaccess® is exceptionally positioned to assist manufacturers in these efforts.
As Dr. Joseph S. Ross emphasized, the participation of manufacturers in after-sales assessments is vital for maintaining public trust and enhancing healthcare outcomes.
Navigating Brazil's Regulatory Framework for Post-Market Trials
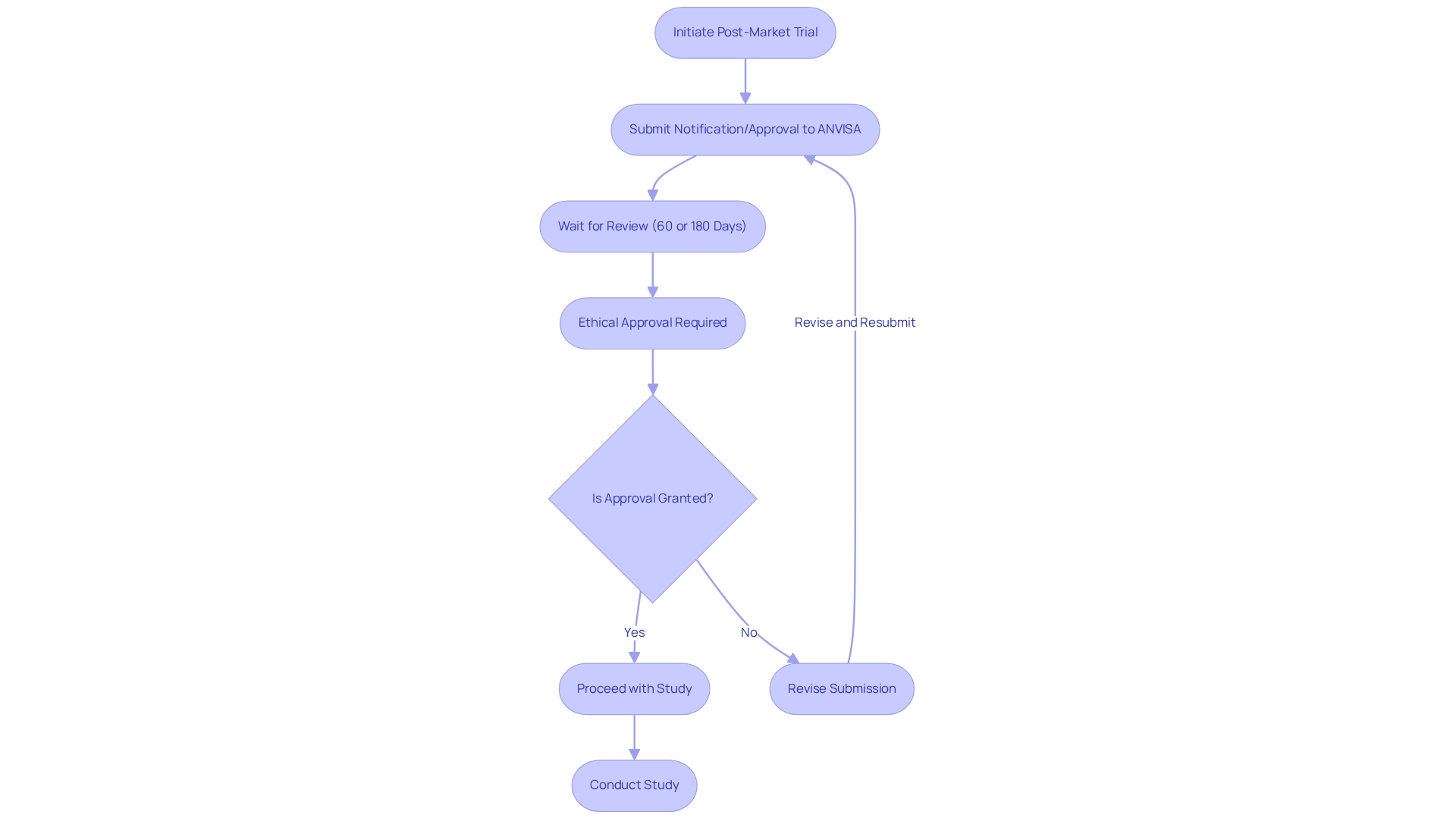
In Brazil, the National Health Surveillance Agency (ANVISA) plays a vital role in overseeing medical studies, particularly focusing on post-market trial design. The recent implementation of Resolution No. 945/2024 has greatly simplified the approval procedure for after-market studies, providing researchers and sponsors enhanced flexibility in study design.
This resolution mandates that any post-marketing variations require either notification or approval from ANVISA, with review times set at sixty days for priority applications and 180 days for ordinary submissions. Notably, ANVISA accepts a truncated approach (AUC72) for products with a long half-life of more than 24 hours, further illustrating the agency's commitment to regulatory flexibility.
It is essential for researchers and sponsors to fully comprehend these regulations, which encompass the requirement for ethical approvals and the submission of research notifications. Familiarity with these requirements not only ensures compliance but also enhances the overall quality and credibility of the research conducted. For instance, studies involving indigenous populations must adhere to specific ethical guidelines that respect their cultural and social contexts.
Investigators are required to promote the well-being of indigenous participants while ensuring that the research benefits their communities, demonstrating a commitment to ethical research practices.
As of 2025, revisions to ANVISA's after-market research regulations continue to develop, demonstrating a dedication to enhancing the research environment in Brazil. Successful approvals following market evaluations under Resolution No. 945/2024 exemplify the positive impact of these regulatory changes, showcasing how they facilitate timely access to innovative medical technologies.
Specialists in the area stress that grasping Brazil's regulatory structure for research is crucial for the post-market trial design, enabling efficient management of the intricacies of after-sales monitoring and adherence. With over 20 years of experience in Medtech, bioaccess® is well-positioned to guide you through these regulatory landscapes, leveraging our specialized knowledge and flexibility in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies. As Negar Gharavi, Senior Director of Medical Writing & Regulatory Affairs, mentions, "BioPharma Services can assist in steering your next pharmaceutical drug development project from concept to clinic," emphasizing the significance of expert guidance in this changing regulatory landscape.
Challenges in Designing Effective Post-Market Trials in Brazil
Creating efficient post-market trial design for Brazil requires navigating a landscape filled with challenges. A primary concern is patient recruitment, particularly given the country's diverse population, which can lead to significant variability in responses to medical devices. Recent statistics reveal that less than 10% of ongoing oncology clinical studies are accessible in Latin America, underscoring the difficulties in involving patients in clinical research.
These challenges are compounded by logistical issues, such as the need to coordinate with various research locations and ensure adherence to local regulations, complicating the implementation of studies. Furthermore, the integrity of data collection is paramount; ensuring that data is gathered consistently and accurately across different locations is essential for maintaining the study's validity. Expert insights indicate that while regulatory bodies such as ANVISA and CONEP have made strides in enhancing the research environment, obstacles remain due to prolonged approval procedures and insufficient governmental funding, particularly for cancer research. A study titled "Barriers to Cancer Research in Brazil" highlights that these regulatory challenges and funding issues significantly hinder Brazilian involvement in research, limiting the capacity to conduct relevant studies and restricting patient access.
In this context, partnerships like that of bioaccess™ with GlobalCare Clinical Trials are vital. Their collaboration has led to improved ambulatory services for research in Colombia, achieving over a 50% reduction in recruitment duration and 95% retention rates. Such initiatives are crucial for addressing the complexities of the regulatory environment and financial constraints, as emphasized by Carlos H Barrios, who asserts that "the intricacies of the regulatory environment and financial constraints must be navigated carefully to improve involvement in research studies."
Moreover, efforts are underway to enhance the infrastructure of clinical research locations in isolated and underserved regions of Brazil, which could positively influence patient recruitment and study execution. Tackling these multifaceted challenges necessitates meticulous planning and collaboration among all stakeholders involved. By crafting robust study designs that account for these complexities, researchers can enhance the likelihood of successful outcomes in post-market trial design for Brazil, ultimately facilitating the advancement of medical devices in the Brazilian market.
Collaborative Approaches: Engaging Stakeholders in Trial Design
Involving stakeholders in the development of post-market studies is essential for achieving positive outcomes. This process necessitates collaboration with healthcare professionals, regulatory authorities, and patient advocacy groups. By engaging these stakeholders early in the study design, researchers can ensure that the investigation effectively addresses real healthcare needs and complies with regulatory standards.
Effective communication and collaboration not only streamline the approval process but also enhance participant recruitment efforts. To foster meaningful engagement, strategies such as stakeholder workshops and feedback sessions can be implemented. These approaches facilitate the collection of valuable insights and promote a sense of ownership among all parties involved. For instance, GlobalCare Clinical Studies' recent collaboration with bioaccess™ to expand research ambulatory services in Colombia demonstrated that involving healthcare professionals in study design significantly improved the relevance of study parameters, resulting in a reduction in recruitment duration by over 50% and a retention rate exceeding 95%.
Furthermore, statistics indicate that projects with active stakeholder involvement experience a 30% increase in success rates compared to those without such collaboration. This underscores the critical role that stakeholder participation plays in enhancing the overall efficiency of clinical studies, particularly concerning post-market trial design in Brazil's evolving Medtech landscape. Additionally, the Flesch-Kincaid Grade Level for the Participant Consent Form was determined to be 12.0, highlighting the importance of readability in ensuring that all stakeholders can effectively engage with study materials.
A case study titled 'Improving Patient Engagement through Readability' illustrates that complex language can impede understanding and participation among subjects. By employing readability formulas and visual aids, researchers can improve patient comprehension and engagement, ultimately leading to a more informed consent process. By prioritizing these collaborative strategies, researchers can adeptly navigate the complexities of post-market trial design in Brazil, contributing to the advancement of medical devices that improve patient outcomes.
Moreover, bioaccess™ provides comprehensive clinical study management services, including feasibility studies, site selection, compliance evaluations, study setup, import permits, project management, and reporting, which are vital for the success of clinical studies. As emphasized by experts in the field, "All authors agree to be accountable for all aspects of this work," underscoring the collective responsibility in achieving successful study outcomes.
Expert Insights: Best Practices for Post-Market Trial Design
Creating effective post-market trial design for Brazil necessitates adherence to several best practices that significantly enhance credibility and impact. First and foremost, it is essential to clearly define the study objectives, ensuring alignment with regulatory requirements established by authorities such as INVIMA, Colombia's National Food and Drug Surveillance Institute. This alignment facilitates compliance and approval, which is crucial for navigating the complex Medtech landscape.
Employing adaptive study designs is another essential strategy; these designs allow for adjustments based on interim findings, thus enhancing flexibility and responsiveness to emerging data. As Dr. Tao Chen, a senior lecturer in Biostatistics, observes, "Statistics, including its use in research design and analysis, is consistently developing with continuous technological progress." This adaptability is particularly important in the dynamic landscape of medical device studies, where initial findings can inform subsequent phases of research.
Moreover, prioritizing patient engagement is vital. Incorporating patient feedback into the study design not only boosts recruitment and retention rates but also ensures that the research addresses real-world concerns and needs. This method fosters a more patient-focused research setting, increasingly recognized as an optimal practice in clinical studies.
Strong information management practices are also essential for enabling precise and prompt collection and analysis. For instance, to detect the effect size at 425 grams, a total of 296 animals are required, underscoring the importance of statistical considerations in trial design. Efficient information management ensures that researchers can draw trustworthy conclusions from their studies, ultimately leading to improved decision-making and outcomes.
Additionally, employing methods such as multiple imputation can help manage missing data, further bolstering data integrity.
By applying these optimal approaches, such as utilizing adaptive study designs and emphasizing patient involvement, researchers can significantly enhance the quality and efficacy of their evaluations through post-market trial design for Brazil. This approach not only aligns with current regulatory expectations but also positions studies for greater success in the evolving Medtech landscape, particularly with the expertise of bioaccess® in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies. Furthermore, as highlighted in the LATAM Medtech Leaders Podcast, understanding local market dynamics and engaging with regional stakeholders can provide invaluable insights that enhance study results and market access strategies.
Data-Driven Decisions: The Role of Data Collection in Post-Market Trials
Data collection serves as the backbone of the post-market trial design for Brazil, delivering essential evidence to evaluate the ongoing safety and effectiveness of medical devices. To achieve robust outcomes, researchers must adopt comprehensive information collection strategies that encompass both quantitative and qualitative methods. The integration of electronic information capture (EDC) systems is especially advantageous, as these technologies simplify information management procedures and considerably improve accuracy.
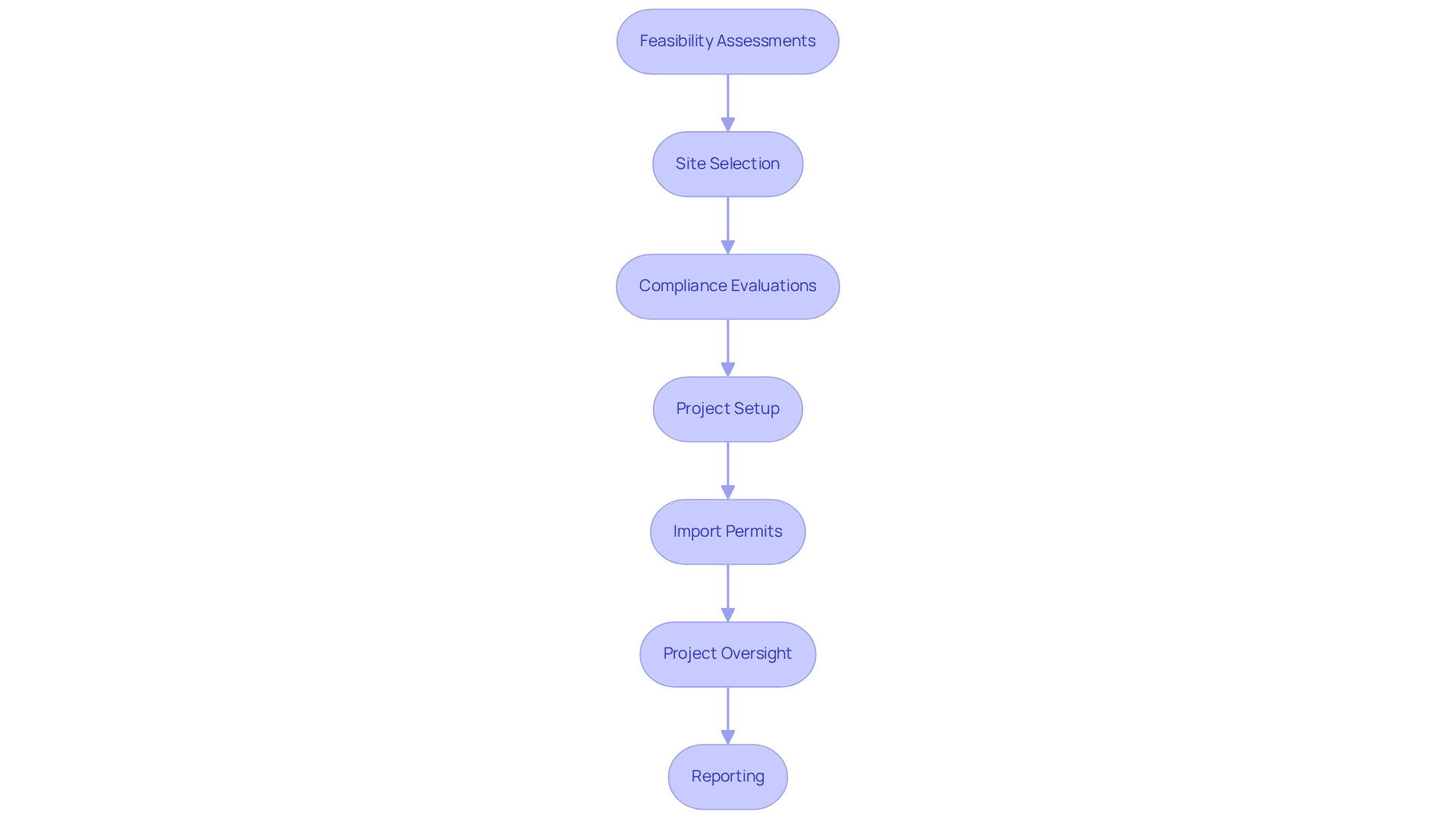
For example, EDC systems can enable real-time information entry and supervision, decreasing the chances of mistakes and enhancing the dependability of results. At bioaccess®, we utilize our 20+ years of experience in overseeing research studies to guarantee that our clients gain from extensive management services for research projects, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Project setup
- Import permits
- Project oversight
- Reporting
Our specialized focus includes Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and also the Post-market trial design for Brazil along with Post-Market Clinical Follow-Up Studies (PMCF). Establishing clear protocols for information monitoring and analysis is crucial for promptly identifying trends and potential safety signals.
This proactive approach allows researchers to respond swiftly to emerging issues, thereby safeguarding patient safety and maintaining compliance with regulatory standards. The SPIRIT and CONSORT guidelines offer crucial suggestions for study protocols and reporting, ensuring clarity and reproducibility in clinical studies. Moreover, network plots can be utilized to summarize connections between reported events, offering insights into the strength of associations and assisting in the interpretation of intricate information sets. Considering the difficulties related to spontaneous reporting systems—like underreporting and overreporting of adverse events, as noted in recent case studies—implementing organized information collection methods becomes even more essential. These methods not only enhance the reliability of safety signals but also contribute to a more comprehensive understanding of the medical device's performance in real-world settings, especially in the context of post-market trial design for Brazil. As S Schneeweiss noted, in the absence of sufficient direct effectiveness studies, comparative effectiveness research is crucial for addressing the limitations of generalizability to routine care.
By prioritizing data-driven decision-making, researchers can ensure that their findings significantly enrich the existing body of evidence surrounding medical devices, ultimately fostering innovation and improving patient outcomes. For instance, in an experiment for sitravatinib, the determination of the optimal 120 mg dose was based on trade-off desirability scores, illustrating the importance of data in making informed decisions regarding dosage and treatment efficacy. Our tailored method at bioaccess® guarantees that we manage the intricacies of medical studies in Latin America efficiently.
Future Trends: Innovations in Post-Market Trial Design
The landscape of post-market evaluations in Brazil is undergoing significant transformation, driven by various emerging trends. A pivotal advancement is the increasing reliance on real-world evidence (RWE) to augment traditional research information. This integration offers a more nuanced understanding of a medical device's performance across diverse patient demographics, thereby enhancing the overall quality of evidence available to stakeholders.
A case study titled 'Real-World Information Applications in Clinical Studies' investigates the application of real-world information (RWI) in clinical studies, revealing that out of 2020 screened articles, only 89 met the criteria for analysis. This statistic underscores the underutilization of RWI in medication or procedure studies, highlighting the necessity of incorporating RWE into post-market studies to tackle existing challenges.
Moreover, advancements in technology, particularly in artificial intelligence (AI) and machine learning, are revolutionizing information analysis and refining experimentation processes. Kristin Kostka, Director of the OHDSI Center at Northeastern University’s Roux Institute, notes, "There’s more information being gathered than we ever thought humanly possible." These innovations not only enhance the efficacy of research studies but also facilitate more comprehensive data analysis, enabling quicker adjustments to study designs based on real-time insights.
As regulatory bodies adapt to these advancements, it is imperative for researchers to remain agile and proactive in adopting new methodologies. The future of post-market trial design in Brazil will likely hinge on the successful integration of RWE and advanced technologies, ultimately leading to more effective and adaptable research practices. bioaccess® stands ready to support these initiatives with comprehensive research project management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project oversight
- Reporting
Continued exploration of appropriate uses of real-world data (RWD) is essential to enhance its application in clinical trials and ensure its suitability for use.
Conclusion
The exploration of post-market trials in Brazil underscores their pivotal role in ensuring the ongoing safety and effectiveness of medical devices. These trials not only identify rare adverse events and assess long-term device performance but also align with regulatory requirements, thereby fostering public trust. Recent regulatory changes, particularly through ANVISA, have streamlined the approval process, facilitating navigation through the complexities of post-market studies for researchers and sponsors alike.
Nonetheless, challenges persist, especially in patient recruitment and data collection integrity. Collaborative approaches that engage stakeholders throughout the trial design process are vital for overcoming these obstacles. By integrating feedback from healthcare professionals and patient advocacy groups, researchers can develop trials that are both relevant and effective. Best practices, such as adaptive trial designs and a strong emphasis on patient engagement, further enhance the credibility and impact of post-market studies.
Looking forward, the integration of real-world evidence and advancements in technology, including artificial intelligence, are poised to revolutionize post-market trial methodologies. Embracing these innovations will not only improve trial efficiency but also enrich the quality of data collected, ultimately leading to superior healthcare solutions. As Brazil's medical device landscape continues to evolve, a steadfast commitment to robust post-market trials will be essential for sustaining public confidence and advancing patient care.
Frequently Asked Questions
What are post-market studies in the context of medical devices?
Post-market studies are evaluations conducted after medical devices have entered the market to assess their safety and efficacy. They help identify rare adverse events, evaluate long-term effectiveness, and provide insights for future regulatory decisions.
Why are post-market studies particularly important in Brazil?
In Brazil, the medical device market is rapidly expanding, making post-market trial design crucial for manufacturers and regulatory authorities. These studies ensure compliance with local regulations, foster public confidence in medical technologies, and enhance market competitiveness.
What recent developments have been made regarding post-market studies in Brazil?
The implementation of Resolution No. 945/2024 by ANVISA has simplified the approval process for post-market studies, allowing for greater flexibility in study design and establishing specific review times for applications.
What are the review times for post-marketing variations under ANVISA's new resolution?
Review times are set at sixty days for priority applications and 180 days for ordinary submissions.
What ethical considerations must researchers keep in mind when conducting studies involving indigenous populations in Brazil?
Researchers must adhere to specific ethical guidelines that respect the cultural and social contexts of indigenous populations, ensuring the well-being of participants and that the research benefits their communities.
How does bioaccess® support manufacturers in post-market studies?
Bioaccess® offers expertise in managing follow-up studies post-market release, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, helping manufacturers navigate regulatory challenges effectively.
What is the significance of ongoing vigilance in post-market evaluations?
Ongoing vigilance is crucial as a substantial proportion of adverse events occur post-launch, highlighting the importance of continuous monitoring to ensure device safety and effectiveness in real-world settings.
How do post-market studies contribute to the improvement of medical devices?
After-market trials have led to enhancements in device safety and effectiveness, demonstrating their critical role within the regulatory framework and ensuring that devices maintain their performance over time.




