Introduction
In the realm of healthcare, the integrity of medical device packaging is not merely a logistical concern; it is a critical component of patient safety and care quality. As the stakes rise with the potential for contamination, understanding the complexities of sterility becomes essential for manufacturers and healthcare providers alike.
From stringent regulatory standards to innovative sterilization technologies, the landscape of sterile packaging is continuously evolving, reflecting the dual pressures of compliance and the imperative to protect patient health.
This article delves into the multifaceted aspects of sterile medical device packaging, exploring best practices, emerging challenges, and the pivotal role of quality control measures in safeguarding against contamination.
Through a detailed examination of current innovations and industry standards, it aims to illuminate the best pathways for ensuring that medical devices remain safe and effective from the moment they are packaged until they are used in critical healthcare settings.
The Importance of Sterility in Medical Device Packaging
Guaranteeing sterility in sterile medical device packaging is essential for preventing contamination and ensuring patient health. Contaminated equipment can lead to severe infections, complications, and even fatalities. The importance of strict sterility standards is emphasized by regulations like the FDA guidelines, which require that sterile medical device packaging ensures instruments are free of microorganisms before patient use.
For example, a lapse in sterility during surgical procedures can result in devastating post-operative infections, significantly hindering patient recovery and exposing healthcare providers to potential legal ramifications. As Peter H. Nibbering, Associate Professor at the Leiden University Medical Center, emphasizes, 'Understanding pathogen-host interactions is crucial in developing effective sterilization methods.' Recent data highlighting that Staphylococcus aureus, Pseudomonas spp, and Klebsiella spp are among the most frequently identified pathogens in outbreaks—accounting for 14.8%, 8.9%, and 7.1% of cases respectively—underscores the stakes involved.
The implications of ineffective sterile practices are further illustrated by the case of Staphylococcus epidermidis, an opportunistic pathogen responsible for a large percentage of catheter-associated bloodstream infections (CA-BSIS) and exhibiting over 70% methicillin resistance. This situation illustrates the essential aspect of sterility; the pathogen's capacity to create biofilms on healthcare tools complicates treatment alternatives and raises costs. Therefore, understanding and implementing robust protocols for sterile medical device packaging is not merely a regulatory obligation; it is a fundamental aspect of patient safety and quality care.
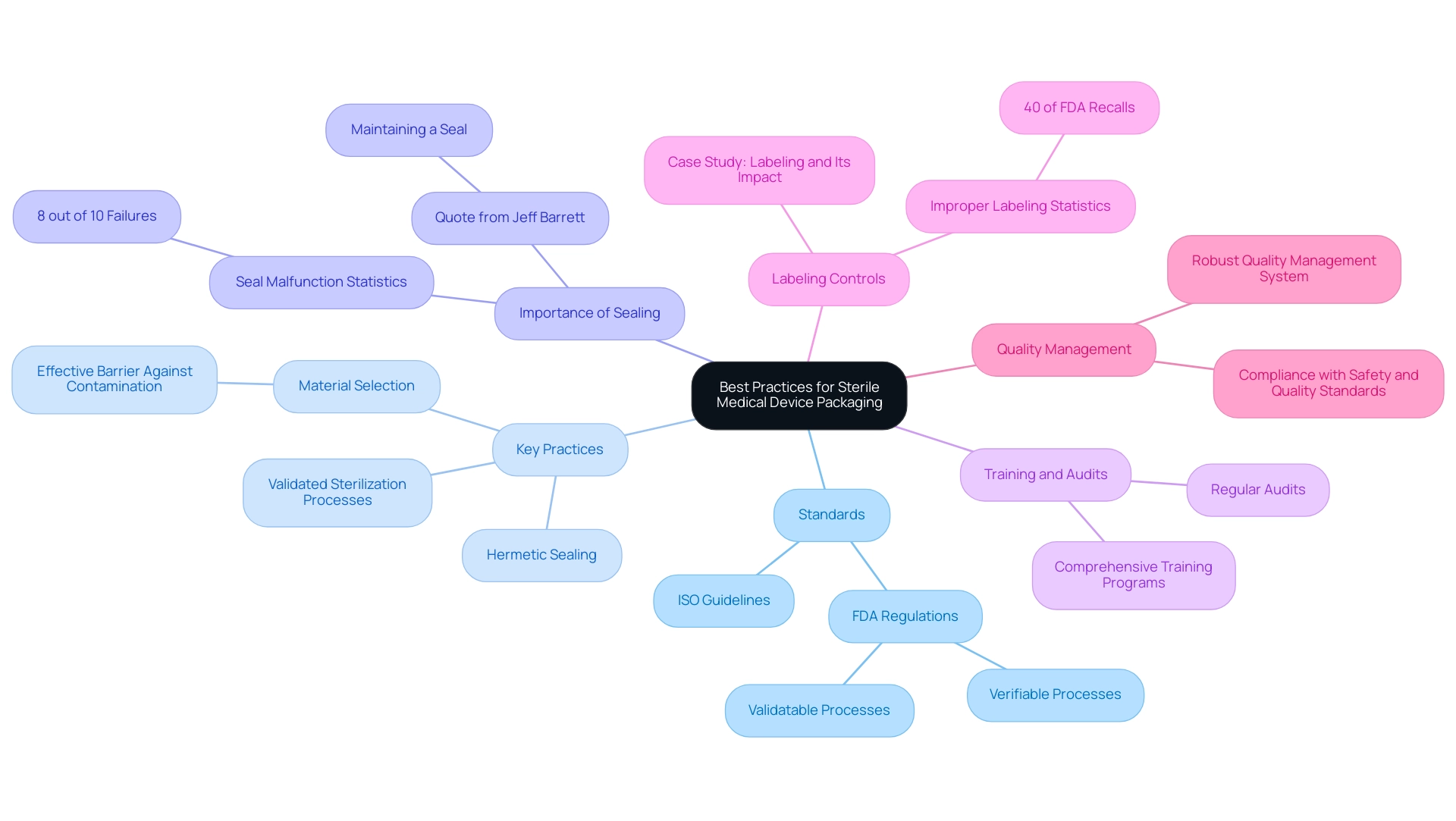
Best Practices and Standards for Sterile Medical Device Packaging
Adhering to best practices and standards is crucial for ensuring the integrity of sterile medical device packaging. Organizations must align with guidelines set by authorities such as the International Organization for Standardization (ISO) and the FDA. Key practices for sterile medical device packaging include:
- Employing validated sterilization processes
- Selecting materials that provide an effective barrier against contamination
- Ensuring hermetic sealing of all containers
The importance of maintaining a proper seal is echoed by Jeff Barrett, CEO of J-Pac Medical, who states,
It's complicated. But in the end, it's all about maintaining a seal and not damaging the product.
Significantly, in 8 out of 10 instances regarding these failures, it’s the product and the seal malfunctioning, not the product breaking, highlighting the importance of container integrity.
Regular audits and comprehensive training programs are essential to ensure that staff members are well-versed in the processes related to sterile medical device packaging and regulatory requirements. Additionally, improper labeling has emerged as a leading cause of FDA recalls, accounting for 40% of all incidents, as highlighted in the case study titled 'Labeling and Its Impact on FDA Recalls.' This highlights the critical need for strong labeling controls throughout the wrapping process.
By implementing a robust quality management system, organizations can enhance compliance and ensure that their processes consistently meet safety and quality standards, ultimately safeguarding both the product and the patient. Furthermore, with evolving standards and practices in the industry, as discussed in the recent article 'What Does 2025 Hold for Medical Device Manufacturers?', staying informed is vital for continued compliance and improvement.
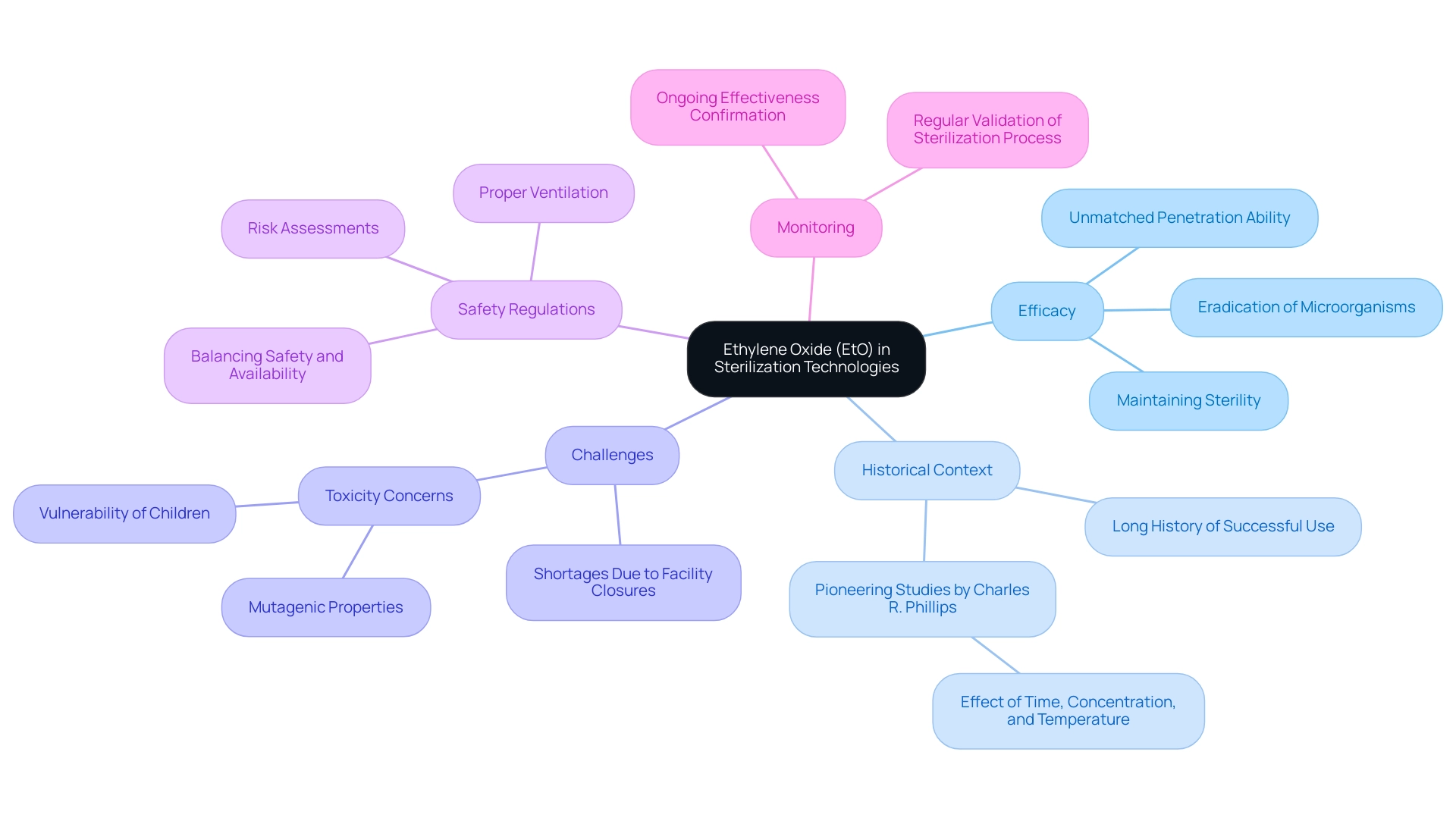
Understanding Sterilization Technologies: The Role of Ethylene Oxide
Ethylene Oxide (EtO) acts as an essential disinfection method for heat-sensitive instruments, showcasing unmatched efficiency in penetrating packaging materials to eradicate bacteria, viruses, and fungi, thus guaranteeing that instruments maintain sterility until required. Notably, approximately 50 percent of sterile medical device packaging, amounting to around 20 billion annually, are treated using this gas. By the 1940s, pioneering studies conducted by Charles R. Phillips elucidated the disinfecting capabilities of EtO, emphasizing the significance of time, concentration, and temperature in the process.
While its efficacy is well-established, challenges have arisen due to recent shortages caused by the closures of EtO sanitation facilities, posing significant hurdles for the industry. Additionally, EtO presents challenges related to toxicity, necessitating rigorous risk assessments, proper ventilation, and adherence to safety regulations during its application. Views on these regulations differ, emphasizing the necessity for a balanced strategy to ensure safety without undermining the availability of essential sanitation services.
Furthermore, organizations must prioritize regular monitoring and validation of the sterilization process to confirm its ongoing effectiveness. A thorough comprehension of these factors is essential in maintaining safety standards in the production of healthcare tools, particularly considering recent worries about the possible vulnerability of children to the mutagenic impacts of EtO exposure due to their developing bodies. The case study entitled 'Why ETO is Uniquely Effective' further demonstrates that Ethylene Oxide is crucial for sterilizing a broad array of sterile medical device packaging, emphasizing its distinctive characteristics that contribute to its enduring success.
Quality Control Measures in Sterile Medical Device Packaging
Quality control measures are essential in ensuring sterile medical device packaging, as they verify that packaging processes meet regulatory compliance and effectiveness standards. Organizations should adopt a comprehensive quality assurance program that includes regular testing of materials to assess sterility and integrity. Technologies such as 2-D barcodes with unique numbering/serialization, UV identification codes, and holograms are crucial in safeguarding against product counterfeiting.
Conducting validation studies is vital to ensure that sterilization processes are both effective and reproducible. Furthermore, routine inspections and audits of the wrapping process play a vital role in identifying and correcting potential issues before they compromise product safety. It is important for customers to indicate any special conditions on the submission form and to familiarize themselves with the peel study SOP to ensure compliance with regulatory requirements.
As mentioned by Van der Stahl Scientific, the reputational damage that can arise from labeling errors can be long-lasting and challenging for businesses to recover from. By adhering to stringent quality control measures in sterile medical device packaging, organizations can significantly mitigate the risk of contamination, thereby safeguarding patient safety and maintaining trust in their products. A practical example of effective quality control measures can be seen in the Limited Service Warranty provided by Van der Stahl Scientific, Inc., which covers new machinery for one year from the date of purchase, ensuring it is free from defects in materials and workmanship.
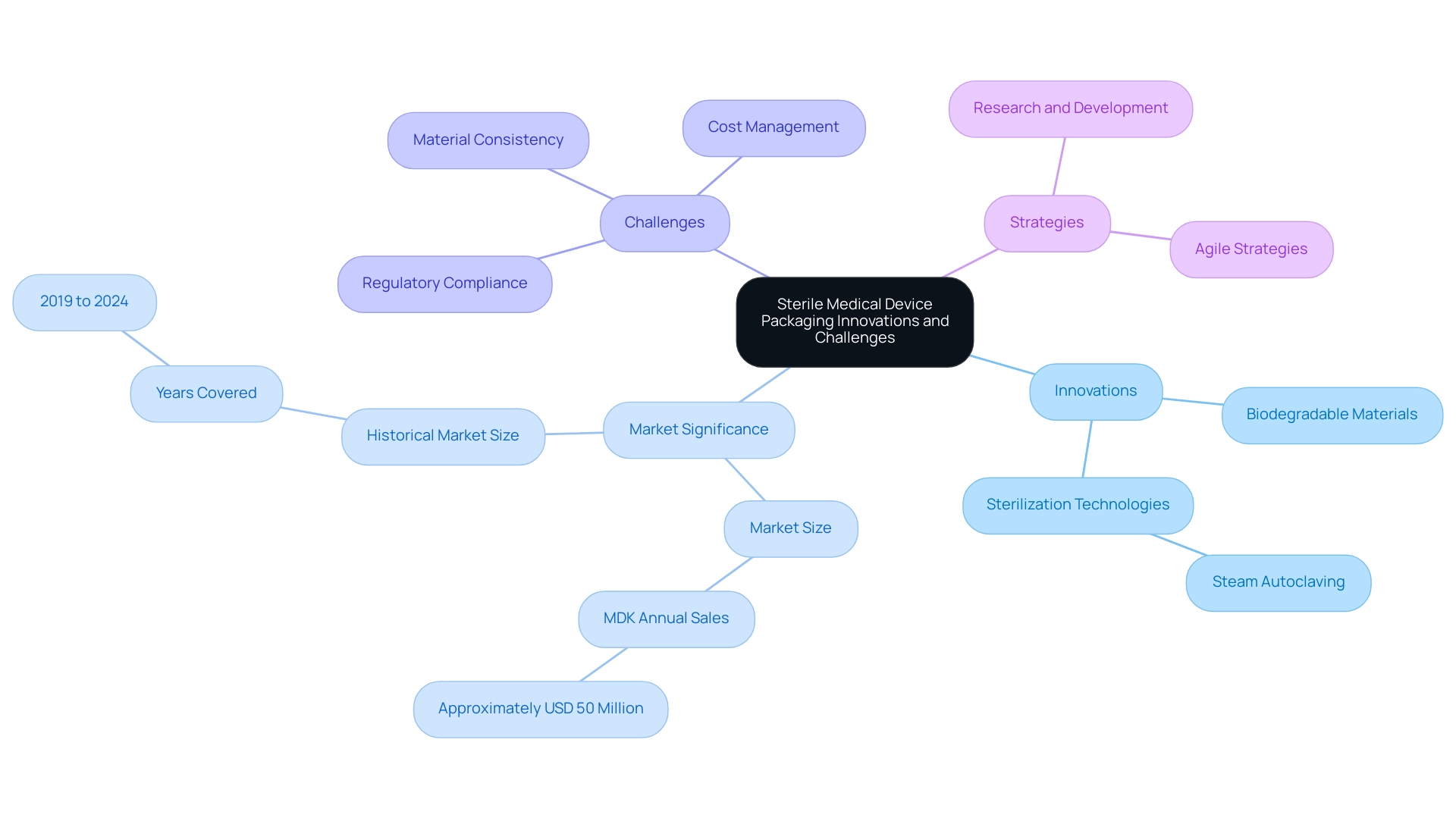
Innovations and Challenges in Sterile Medical Device Packaging
The terrain of sterile healthcare product wrapping is experiencing substantial change, propelled by advancements aimed at improving safety, adherence, and ecological sustainability. Recent advancements have introduced biodegradable materials, which not only help reduce environmental impact but also align with the increasing regulatory focus on sustainability. Furthermore, enhanced sterilization technologies, especially steam autoclaving, have surfaced as the most efficient approach for guaranteeing high-temperature and high-pressure sterilization of healthcare instruments.
Notably, the North America Medical Devices Packaging Market has seen a historical market size that underscores the importance of these innovations, with MDK generating annual sales of approximately USD 50 million, highlighting the market's significance. However, organizations are navigating a landscape filled with challenges, including the need to adapt to evolving regulations and managing the rising costs associated with these advanced solutions. Ensuring the consistency and quality of new materials remains a critical concern.
For instance, sterile medical device packaging such as packing pouches are specialized containers designed to safeguard medical devices during distribution, storage, and transport, maintaining product integrity and sterility throughout their lifecycle. To successfully tackle these challenges, organizations must embrace a proactive and agile strategy, investing in research and development to explore innovative container options while maintaining compliance with existing standards. The emergence of tailored container solutions presents a significant opportunity for growth, especially as the healthcare infrastructure expands in emerging economies.
Embracing these innovations while effectively managing compliance challenges is essential for advancing the field of sterile medical device packaging, as it ensures that product integrity and sterility are maintained throughout the lifecycle of medical devices.
Conclusion
Ensuring the sterility of medical device packaging is vital for patient safety and care quality. Adhering to stringent regulatory standards, such as those set by the FDA, is essential to prevent contamination that can lead to serious health complications. The risks posed by pathogens in healthcare settings highlight the need for robust sterile packaging practices.
Key best practices include:
- Validated sterilization processes
- Appropriate packaging materials
- Effective sealing
All of which are crucial in reducing contamination risks. Regular training and audits further ensure compliance, protecting both products and patients.
Innovations in sterilization technologies, particularly Ethylene Oxide, demonstrate significant advancements, though they also present challenges, such as risks associated with its use and recent shortages. Quality control measures are critical for maintaining safety and integrity, with technologies that help combat counterfeiting and ensure compliance.
As the landscape of sterile medical device packaging evolves, embracing innovations while effectively managing compliance challenges is essential. The transition towards biodegradable materials and improved sterilization methods not only addresses environmental concerns but also positions organizations for future growth. Ultimately, a commitment to stringent sterile packaging protocols is crucial for safeguarding patient health and maintaining trust in medical devices throughout their lifecycle.
Frequently Asked Questions
Why is sterility important in medical device packaging?
Sterility in medical device packaging is crucial for preventing contamination, which can lead to severe infections, complications, and even fatalities. Regulations like the FDA guidelines require that sterile packaging ensures instruments are free of microorganisms before patient use.
What can happen if there is a lapse in sterility during surgical procedures?
A lapse in sterility can result in devastating post-operative infections, significantly hindering patient recovery and exposing healthcare providers to potential legal ramifications.
What pathogens are commonly associated with infections due to inadequate sterility?
Common pathogens include Staphylococcus aureus, Pseudomonas spp, and Klebsiella spp, which account for 14.8%, 8.9%, and 7.1% of infection cases, respectively.
What is the significance of Staphylococcus epidermidis in the context of sterility?
Staphylococcus epidermidis is an opportunistic pathogen responsible for a large percentage of catheter-associated bloodstream infections (CA-BSIS) and shows over 70% methicillin resistance, complicating treatment options and increasing costs.
What are some key practices for ensuring sterile medical device packaging?
Key practices include employing validated sterilization processes, selecting materials that provide effective barriers against contamination, and ensuring hermetic sealing of all containers.
Why is maintaining a proper seal important in sterile packaging?
Maintaining a proper seal is essential because, in 8 out of 10 instances of packaging failures, the malfunction originates from the product and the seal rather than the product itself.
What role do audits and training programs play in sterile packaging?
Regular audits and comprehensive training programs are vital to ensure that staff are knowledgeable about the processes related to sterile medical device packaging and regulatory requirements.
What is a significant cause of FDA recalls related to sterile packaging?
Improper labeling has emerged as a leading cause of FDA recalls, accounting for 40% of all incidents, highlighting the need for strong labeling controls throughout the wrapping process.
How can organizations enhance compliance in sterile packaging?
Organizations can enhance compliance by implementing a robust quality management system that ensures processes consistently meet safety and quality standards, ultimately safeguarding both the product and the patient.
Why is it important for organizations to stay informed about evolving standards in the industry?
Staying informed about evolving standards and practices is vital for continued compliance and improvement in sterile medical device packaging.




