Introduction
In the realm of healthcare, the importance of medical device sterilization cannot be overstated. As a critical process designed to eliminate all forms of microbial life, including resilient bacteria and spores, effective sterilization directly impacts patient safety and the efficacy of medical procedures.
With various methods available—ranging from steam and ethylene oxide to radiation—each technique presents unique advantages and challenges tailored to specific applications. As the industry grapples with the emergence of antibiotic-resistant pathogens, the need for rigorous sterilization protocols has never been more urgent.
This article delves into the intricacies of sterilization methods, regulatory frameworks, and emerging trends, providing a comprehensive overview that underscores the ongoing evolution of practices aimed at safeguarding public health in an increasingly complex medical landscape.
Understanding Medical Device Sterilization: An Overview
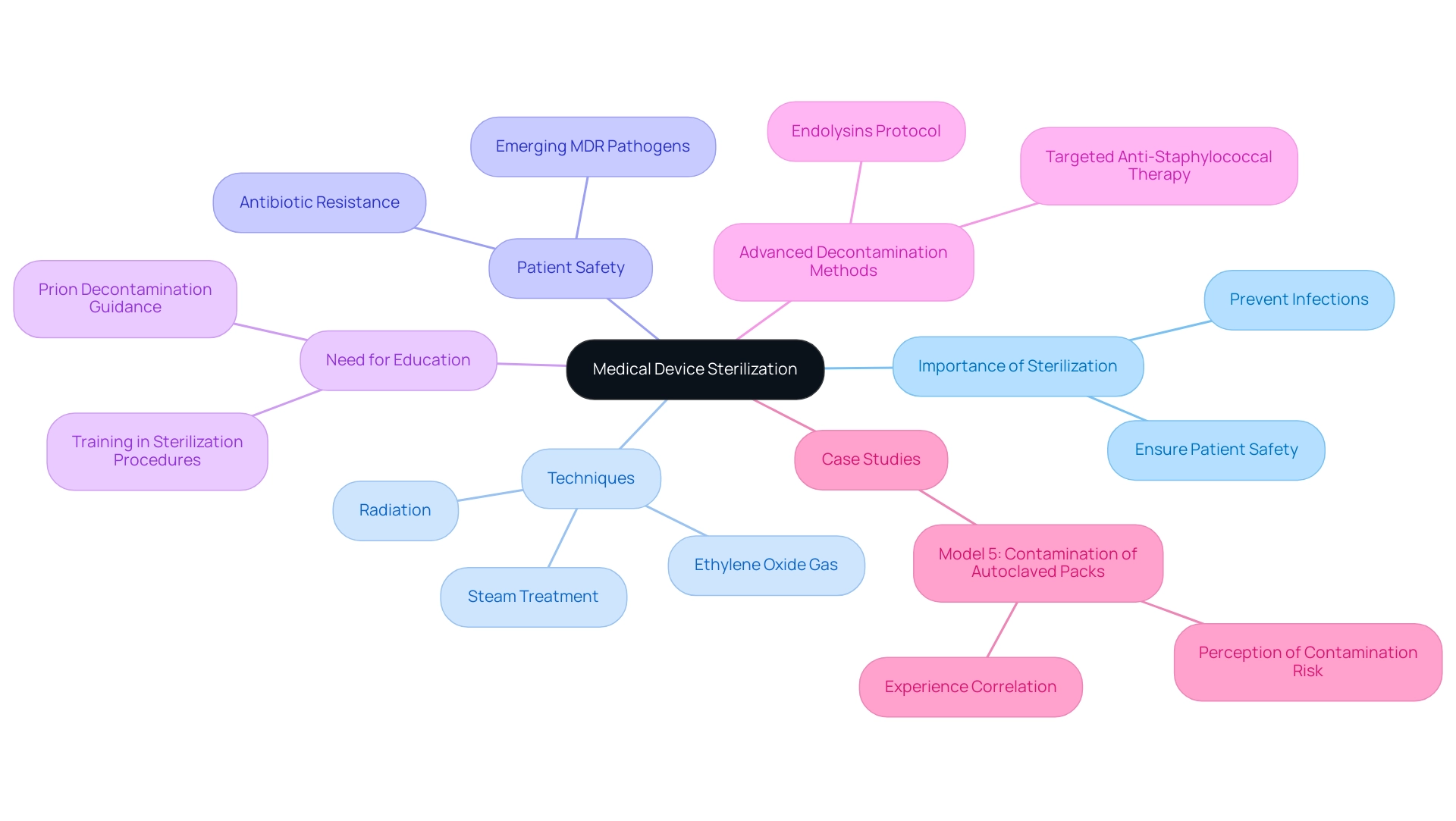
The cleaning of healthcare instruments is crucial, and the sterilization process for medical devices is essential for eliminating all types of microbial existence, such as bacteria, viruses, and spores. With sterility defined as the expectation that fewer than 1 in 1 million devices may harbor bacterial spores, the importance of careful disinfection procedures cannot be overstated. This sterilization process for medical devices is essential to prevent infections and ensure patient safety during healthcare procedures.
Disinfection can be achieved through various techniques, such as:
- Steam treatment
- Ethylene oxide gas
- Radiation
Each technique is customized for particular uses and effectiveness levels. Grasping the principles underlying these sanitation methods is crucial for healthcare professionals, manufacturers, and regulatory bodies alike, as it directly impacts the quality and safety of the sterilization process for medical devices utilized in patient care. As noted by Mary Garvey, Principal Investigator of the PEM Center Team,
In an antibiotic-resistant era of emerging and re-emerging MDR pathogens, it is imperative to ensure patient safety.
This emphasizes the urgent need for strict sanitation protocols in the sterilization process for medical devices. Moreover, a case study titled 'Model 5: Contamination of Autoclaved Packs' revealed that healthcare workers' perceptions of contamination risk correlate significantly with their duration of experience, indicating that longer working periods may heighten concerns regarding the efficacy of the sanitization process. This emphasizes the need for further education and training in hygiene practices, as many healthcare workers have correct knowledge yet still require guidance in specific areas such as sanitation procedures and prion decontamination, as highlighted in recent studies.
Additionally, Totté et al. outlined a study protocol for targeted anti-staphylococcal therapy with endolysins, which highlights the significance of advanced decontamination methods to combat resistant pathogens. Such insights not only reflect the complexities of microbial life removal in healthcare settings but also underscore the ongoing need for comprehensive training to adapt to evolving challenges in disinfection.
Ethylene Oxide Sterilization: Process and Importance
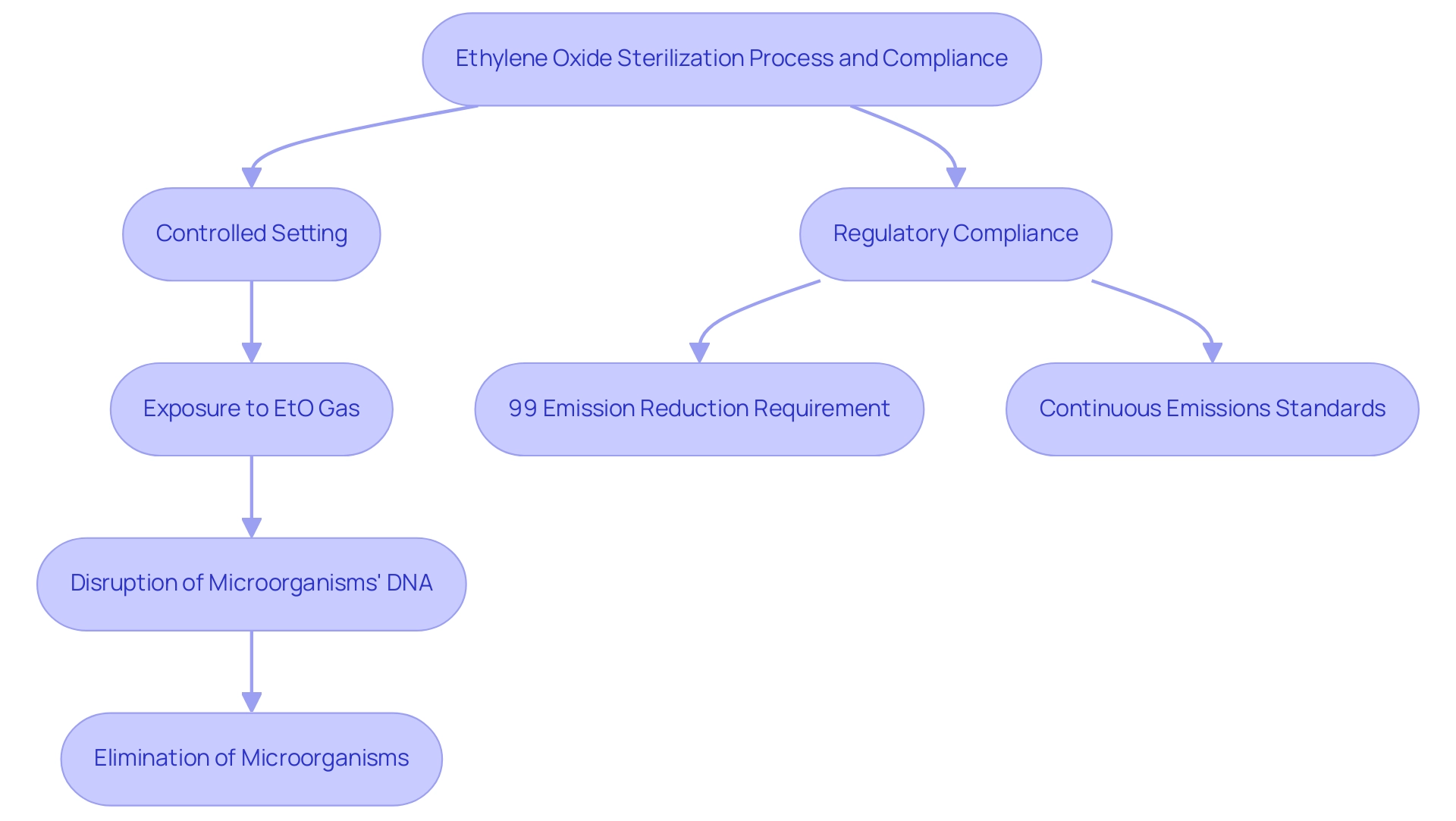
Ethylene Oxide (EtO) treatment is a crucial chemical process in the sterilization process for medical devices that are sensitive to high temperatures or moisture. The disinfection procedure is part of the sterilization process for medical devices, which entails putting the items in a controlled setting where they are subjected to EtO gas. This gas effectively permeates the materials, disrupting the DNA of microorganisms and ensuring their elimination.
This method is particularly vital for delicate instruments, such as those constructed from plastics or containing electronic components, which are commonly found in contemporary healthcare applications. Recent regulations stipulate that for new Sterilization Control Variances (SCVs) initiated after April 5, 2024, facilities using EtO must achieve a continuous 99 percent reduction in emissions upon startup, underscoring the importance of stringent compliance with safety protocols. Furthermore, as noted by the Environmental Protection Agency (EPA), "emissions standards must be continuous in nature," as established in the court ruling related to the Clean Air Act, reinforcing the necessity for constant adherence to safety regulations.
A relevant case study illustrates this point:
- The EPA recalculated the MACT floor for existing Group 1 room air emissions at major source facilities, determining a 90 percent emission reduction based on limited performance data.
- The final MACT floor for existing Group 1 room air emissions was established at 90 percent emission reduction, demonstrating the implications of regulatory compliance.
While EtO disinfection is recognized for its effectiveness, it also necessitates vigilant monitoring due to the potential health risks associated with gas exposure.
The success of EtO in the sterilization process for medical devices underscores its significance in maintaining the safety and efficacy of healthcare technologies.
Comparative Analysis of Medical Device Sterilization Methods
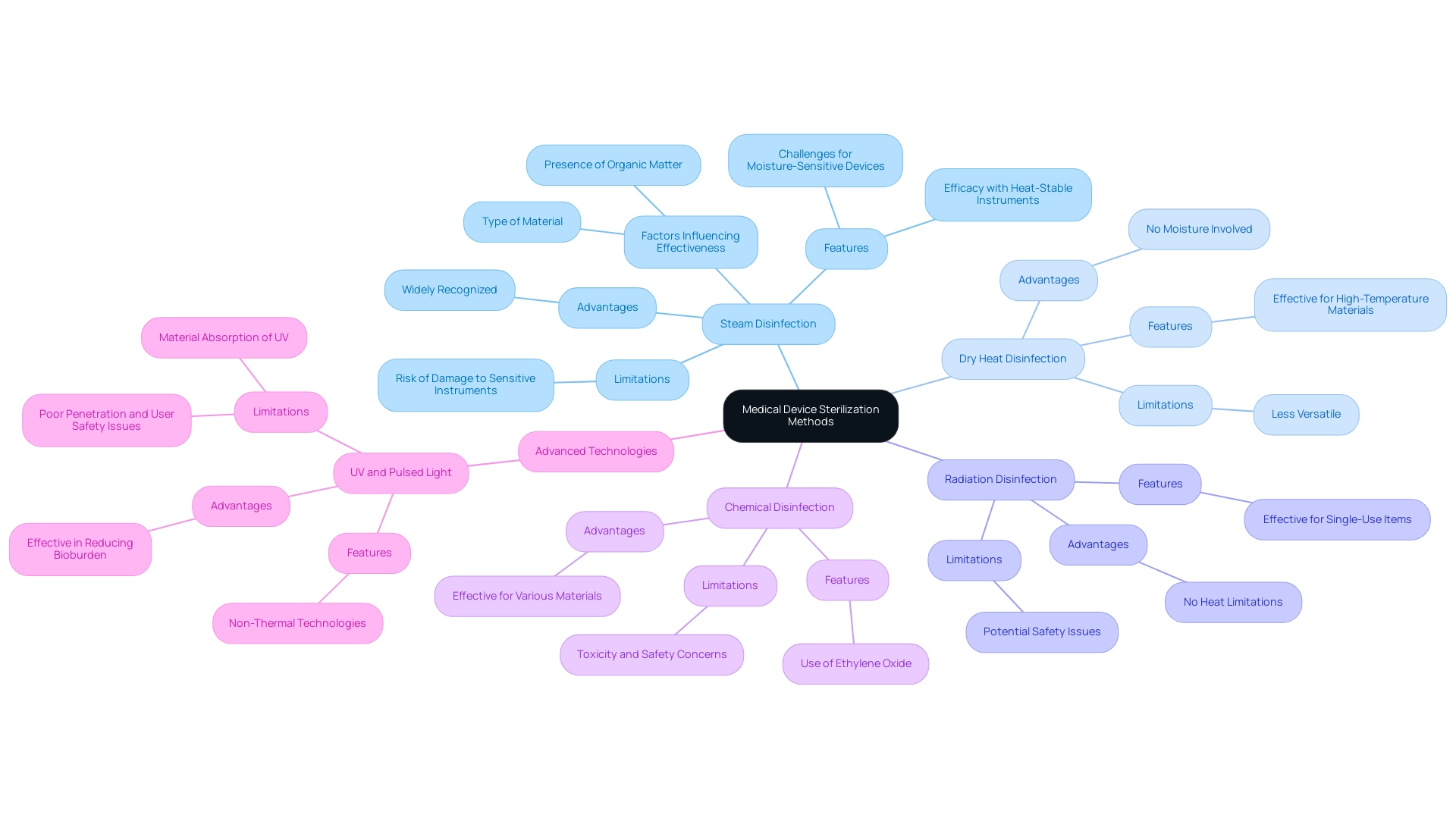
The scenery of the sterilization process for medical devices includes various techniques, each with unique features and uses. Prominent techniques include:
- Steam disinfection (autoclaving)
- Dry heat disinfection
- Radiation disinfection
- Chemical disinfection, such as Ethylene Oxide
Steam disinfection is widely recognized for its efficacy with heat-stable instruments; however, it poses challenges for moisture-sensitive devices due to the risk of damage.
In contrast, radiation disinfection is particularly advantageous for single-use items, providing effective microbial control without the limitations of heat. Dry heat disinfection, while effective, is reserved for materials that can withstand elevated temperatures, making it less versatile than steam.
The effectiveness of each procedure is dependent on several crucial factors, including:
- The type of material
- The presence of organic matter
- The required sterilization process for medical devices for specific applications
Recent literature emphasizes the importance for healthcare professionals to be knowledgeable about these approaches to guarantee the suitable choice of cleaning techniques customized for the instruments in use. For example, recent comparative studies have surfaced, illuminating the relative effectiveness of steam versus radiation methods, especially considering the latest research suggesting differing results based on equipment traits and decontamination contexts.
Furthermore, specialists observe that the selection of the sterilization process for medical devices can greatly influence the overall safety and effectiveness of those devices. As B. P. Rosenbaum highlights, 'the adoption of advanced technologies must also consider privacy and security issues, which can affect the execution of new techniques for disinfection.' This sentiment is emphasized by statistics showing that approximately 30% of healthcare facilities report hesitance in adopting new sanitation technologies due to these concerns.
To further illustrate these points, case studies on advanced decontamination technologies, such as UV and pulsed light, reveal that while these methods are effective in reducing bioburden, they may present challenges such as poor penetration and safety issues related to user exposure. For example, while UV light has shown significant promise in reducing microbial load, its effectiveness is limited by materials like glass and plastics that can absorb UV irradiation. Grasping these nuances is vital for clinical research directors seeking to enhance sanitation protocols in healthcare environments.
Regulatory Framework and Safety Considerations in Sterilization
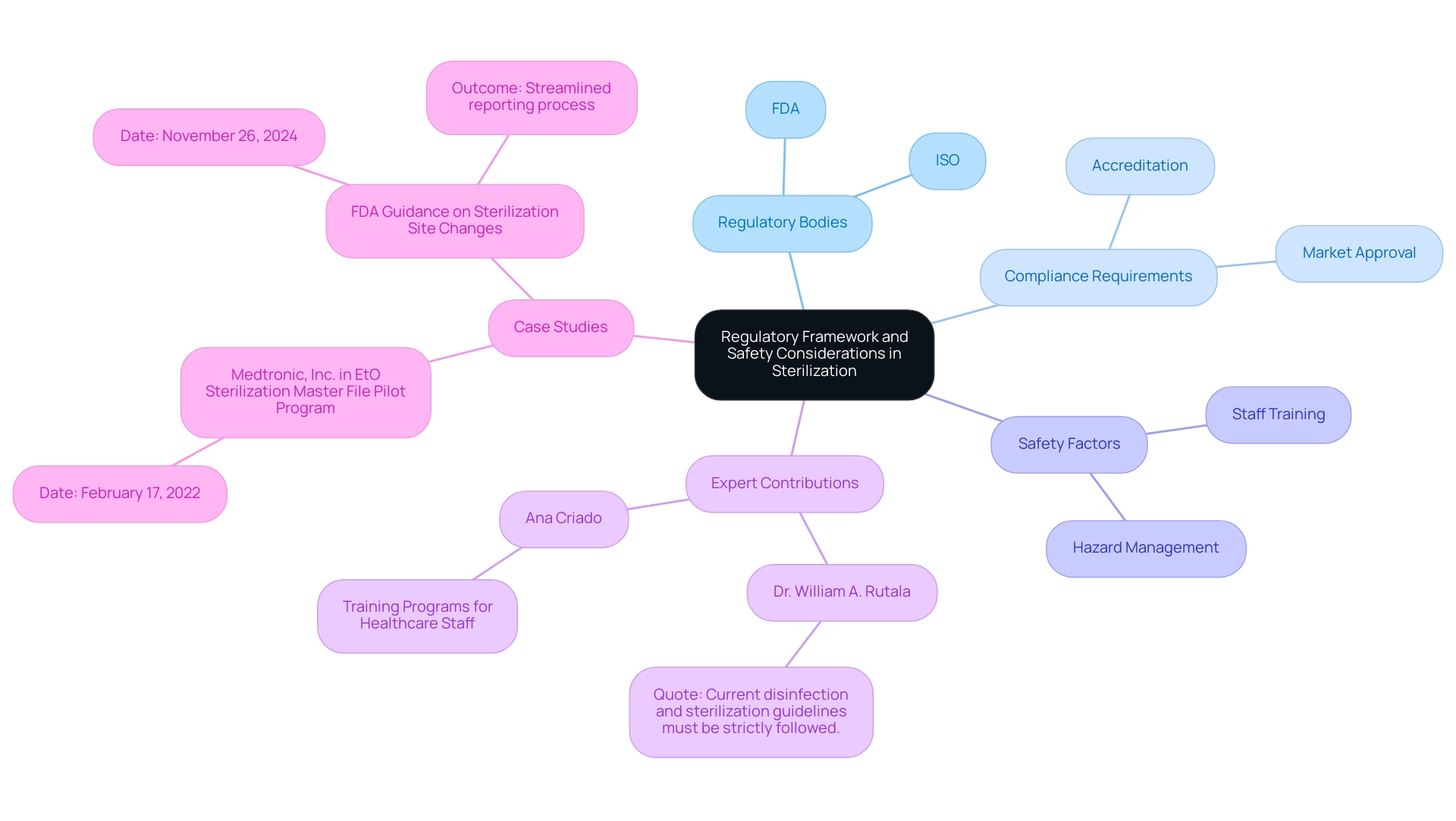
The cleaning of medical devices is supported by strict regulatory frameworks established by the Food and Drug Administration (FDA) and the International Organization for Standardization (ISO). These regulatory guidelines are designed to ensure that disinfection methods adhere to stringent safety and efficacy standards. Compliance with these regulations is not only vital for manufacturers seeking market approval but also for healthcare facilities that must maintain accreditation.
As Dr. William A. Rutala, a specialist in the field, stresses, 'Current disinfection and sanitization guidelines must be strictly followed.' Safety factors in the sanitization procedure encompass the management of sanitized equipment, requiring extensive training for staff to reduce possible hazards. Furthermore, the recent FDA guidance, published on November 26, 2024, regarding the reporting of site modifications for Class III devices, illustrates the agency's commitment to streamlining processes while ensuring compliance during transitions.
This guidance permits manufacturers to report specific sanitation facility changes without prior approval, facilitating smoother operations during times of transition. Regular audits and validation of sanitation processes are imperative to maintain ongoing compliance and effectiveness, with current statistics indicating that adherence to cleaning and disinfection processes remains below 50%. This statistic underscores the critical need for continuous improvement in this vital area.
Additionally, notable experts such as Ana Criado, Director of Regulatory Affairs and consultant for various international companies, emphasize the importance of these standards. Ana's extensive experience in Regulatory Affairs has enabled her to contribute significantly to the development of best practices in sanitation, ensuring that manufacturers not only meet regulatory requirements but also prioritize patient safety. For instance, her participation in training programs for healthcare staff has been crucial in improving adherence to sanitation protocols.
Medtronic, Inc. was accepted into the EtO Sterilization Master File Pilot Program on February 17, 2022, serving as a real-world example of regulatory compliance in action.
Emerging Trends and Future Directions in Medical Device Sterilization
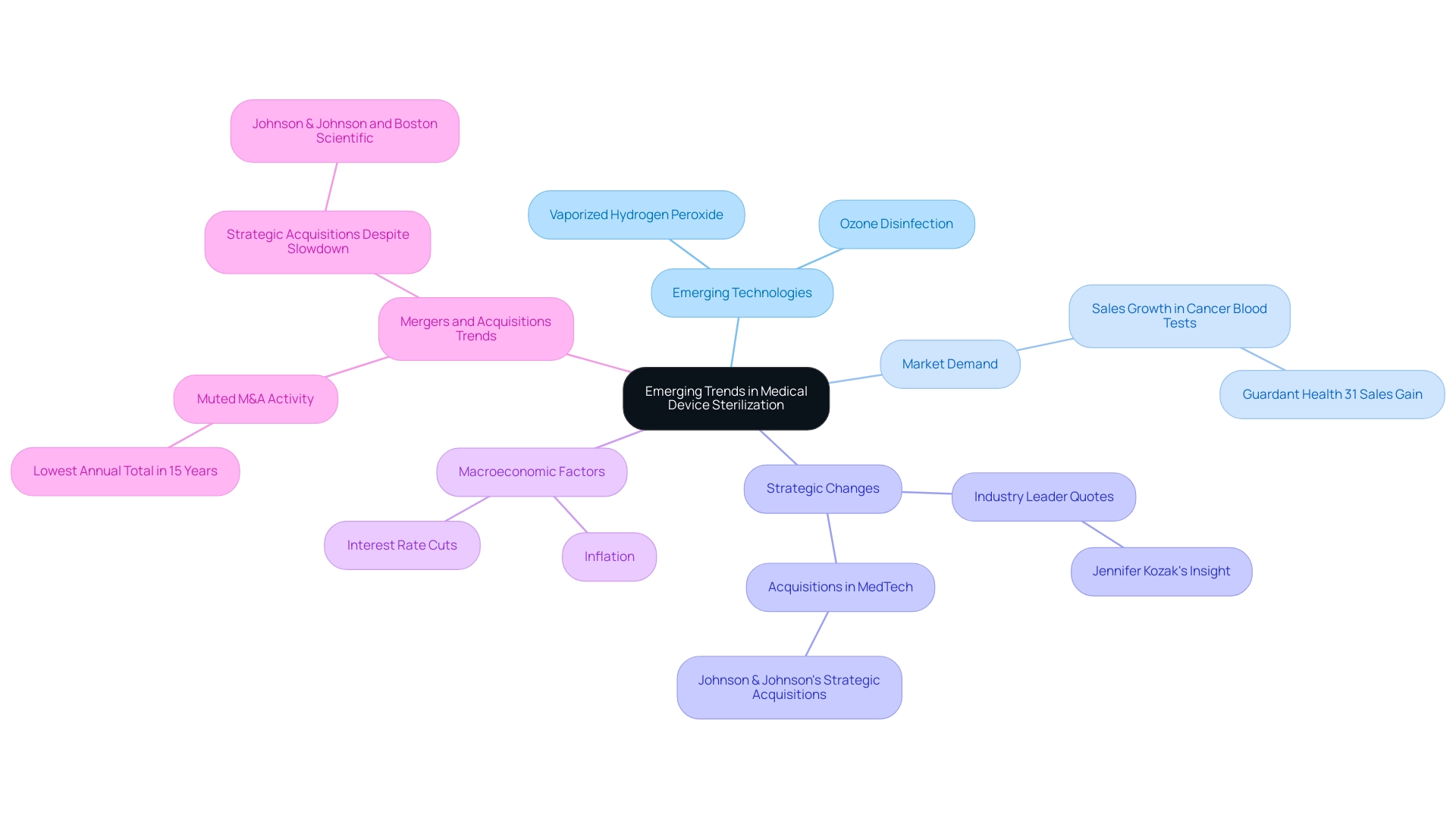
The landscape of medical device disinfection is undergoing significant transformation, with a strong emphasis on the sterilization process for medical devices to enhance both efficiency and effectiveness. Innovations like vaporized hydrogen peroxide (VHP) and ozone disinfection are emerging as viable alternatives to conventional techniques. These advanced technologies not only provide shorter cycle times but also contribute to a reduction in environmental impact, a critical consideration in today's healthcare landscape.
Furthermore, the incorporation of advanced monitoring and validation technologies is essential in guaranteeing dependable and uniform outcomes in the sterilization process for medical devices. As the demand for hygienic medical instruments rises, particularly with the recent increase in sales for cancer blood tests—evidenced by Guardant Health's remarkable 31% sales growth—the continuous research and development of the sterilization process for medical devices becomes increasingly crucial. This evolution is crucial for overcoming the challenges posed by new materials and intricate device designs, thereby preparing the ground for future progress in the sterilization process for medical devices.
As Jennifer Kozak, Vice President of Business Development at Johnson & Johnson, noted, '[the] acquisitions accelerated our ongoing effort to shift into high-growth markets where we feel we have the capabilities that add value and where we can have a leadership position.' This emphasizes the strategic changes in the medtech sector that could affect the acceptance of innovative sanitation technologies. Furthermore, the current slowdown in mergers and acquisitions in the medtech sector, which has seen the lowest annual total in 15 years, indicates a cautious approach that may impact funding in new disinfection techniques.
Coupled with macroeconomic factors such as potential interest rate cuts and inflation, these dynamics are expected to shape the future of the sterilization process for medical devices technologies in 2024.
Conclusion
The exploration of medical device sterilization reveals its critical role in safeguarding patient health and ensuring the efficacy of medical procedures. Various methods, including:
- Steam sterilization
- Ethylene oxide
- Radiation
each present unique advantages and challenges that healthcare professionals must navigate. The importance of adhering to stringent regulatory guidelines established by organizations such as the FDA and ISO cannot be overstated, as these frameworks are essential for maintaining safety and efficacy standards across the industry.
As the healthcare landscape evolves, emerging technologies such as:
- Vaporized hydrogen peroxide
- Ozone sterilization
offer promising alternatives that address both efficiency and environmental concerns. The ongoing development of advanced monitoring and validation systems further enhances the reliability of sterilization outcomes, reflecting the industry's commitment to combatting antibiotic-resistant pathogens and improving patient safety.
The need for comprehensive training and education in sterilization practices remains a priority. With the complexities of microbial life elimination growing, professionals in the field must stay informed and adept at implementing the most effective sterilization techniques. This commitment to continuous improvement is vital, not only for compliance but also for the overall advancement of medical device safety in an increasingly complex healthcare environment. Ultimately, the future of medical device sterilization hinges on innovation, stringent regulatory adherence, and a proactive approach to addressing emerging challenges.
Frequently Asked Questions
Why is the cleaning and sterilization of healthcare instruments important?
The cleaning and sterilization of healthcare instruments are crucial for eliminating all types of microbial existence, such as bacteria, viruses, and spores, which helps prevent infections and ensures patient safety during healthcare procedures.
What is the definition of sterility in medical devices?
Sterility is defined as the expectation that fewer than 1 in 1 million devices may harbor bacterial spores.
What are the main techniques used for disinfection in medical device sterilization?
The main techniques for disinfection include steam treatment, ethylene oxide gas, and radiation, each customized for particular uses and effectiveness levels.
What is the significance of understanding sanitation methods for healthcare professionals?
Grasping the principles underlying sanitation methods is crucial for healthcare professionals, manufacturers, and regulatory bodies, as it directly impacts the quality and safety of the sterilization process for medical devices utilized in patient care.
How do healthcare workers' perceptions of contamination risk relate to their experience?
A case study indicated that healthcare workers' perceptions of contamination risk significantly correlate with their duration of experience, suggesting that longer working periods may heighten concerns regarding the efficacy of the sanitization process.
What is the role of Ethylene Oxide (EtO) in the sterilization process?
Ethylene Oxide (EtO) treatment is a chemical process used for sterilizing medical devices that are sensitive to high temperatures or moisture, effectively eliminating microorganisms by disrupting their DNA.
What are the new regulations regarding EtO emissions for sterilization facilities?
New regulations stipulate that facilities using EtO must achieve a continuous 99 percent reduction in emissions for new Sterilization Control Variances (SCVs) initiated after April 5, 2024.
What must facilities comply with according to the Environmental Protection Agency (EPA)?
Facilities must comply with emissions standards that are continuous in nature, as established in a court ruling related to the Clean Air Act.
What health risks are associated with EtO disinfection?
While EtO disinfection is effective, it necessitates vigilant monitoring due to potential health risks associated with gas exposure.
What does the success of EtO in sterilization highlight?
The success of EtO in the sterilization process for medical devices underscores its significance in maintaining the safety and efficacy of healthcare technologies.




