Overview
Best practices for wearable device clinical trial design are pivotal in ensuring compliance with regulatory standards while significantly enhancing patient engagement through real-time health monitoring. This approach not only addresses the pressing needs of clinical research but also highlights the growing importance of user-friendly devices and transparent data management systems. Such integration is essential, as it not only improves participant adherence but also streamlines the collection of reliable data. This, in turn, fosters more effective clinical research outcomes, ultimately reinforcing the necessity for collaboration and innovation in the Medtech landscape.
Introduction
As the healthcare landscape evolves, wearable devices are emerging as transformative tools in clinical trials, reshaping how researchers gather data and engage participants. These devices, ranging from smartwatches to fitness trackers, facilitate real-time monitoring of health metrics, providing a wealth of information that enhances both data accuracy and patient outcomes.
With a growing emphasis on integrating wearable technology in research, the industry is witnessing a shift towards more efficient trial management and increased participant adherence. However, challenges such as regulatory compliance and data management must be navigated carefully to fully harness the potential of these innovations.
This article delves into the multifaceted role of wearable devices in clinical trials, exploring their impact on data collection, patient engagement, and the future of healthcare research.
Understanding Wearable Devices in Clinical Trials
Wearable devices, including smartwatches and fitness trackers, are fundamentally transforming the landscape of research trials by facilitating continuous monitoring of participants' health metrics. These innovative tools capture vital signs, physical activity, and other health-related information in real-time, offering researchers a comprehensive dataset that was previously challenging to collect. The design of wearable device clinical trials not only enhances information gathering but also promotes improved patient outcomes and more dependable research results.
Recent statistics indicate that 58.1% of trials emphasize the precision and dependability of these devices, underscoring their increasing significance in clinical research. The automation of data capture from these devices significantly reduces the administrative burden on research teams, thereby enhancing overall trial management efficiency. Furthermore, the capability of these devices to offer continuous monitoring correlates with enhanced patient adherence to study protocols.
Participants benefit from real-time monitoring of their health metrics, which may include reminders for medication or appointments, ultimately leading to improved engagement and adherence. Expert opinions stress the importance of enhancing wearable technology to ensure its usability across varied populations, particularly in low-resource settings. This focus on inclusivity is crucial for the future of health research, as it seeks to bridge gaps in information collection and participant representation.
As noted by Thijs et al., the effects of undisclosed algorithms can significantly influence information analysis and standardization, highlighting the necessity for transparency in personal technology.
Moreover, case analyses evaluating the quality of research utilizing personal devices have established a framework for assessing the reliability of results. The Medical Education Research Study Quality Instrument was employed to evaluate the credibility of various research designs, ensuring that conclusions drawn from these trials are based on reliable and high-quality research.
In summary, the design of wearable device clinical trials not only streamlines data collection in clinical research but also enhances patient outcomes, making it a vital component of contemporary clinical trial methodologies. Future research is essential to assess the usability of wearable devices for underrepresented populations, ensuring that advancements in technology benefit all segments of the population.
Enhancing Patient Engagement Through Wearable Technology
The design of wearable device clinical trials is crucial in enhancing patient engagement by providing immediate feedback on health metrics. This feedback is vital for sustaining participant motivation and adherence to study protocols. Devices that monitor heart rate, activity levels, and other vital signs not only motivate participants to stay active but also cultivate a sense of accountability regarding their health management. This exchange of real-time information promotes seamless communication between researchers and participants, facilitating timely updates and reminders that keep participants informed and engaged.
Research indicates that patients who feel a stronger connection to their health data exhibit significantly improved engagement in clinical trials. For instance, studies demonstrate that immediate health feedback can lead to higher compliance rates, with reports suggesting that patient adherence can increase by as much as 30% when utilizing wearable technology. Furthermore, a survey revealed that 29% of respondents felt they lacked the essential information needed to understand the recommended treatments, underscoring the importance of clear communication supported by these devices.
This heightened engagement not only enhances retention rates but also improves the quality of data collected during trials. Additionally, the integration of portable devices into health-related research has been illustrated through various case analyses, particularly regarding the regulatory challenges faced by these devices. The regulation of wearable devices varies, with many consumer products lacking published accuracy information, highlighting the need for a multidisciplinary approach to address the challenges posed by device information in oncology care. The design of wearable device clinical trials, which incorporates devices such as smartwatches for continuous monitoring, has shown improved participant retention and satisfaction.
As noted by Samme Xie, "Wearable technology has tremendous potential to provide more significant information gathering to trial studies." As the landscape of medical research evolves, the importance of prompt feedback from wearable device clinical trial designs becomes increasingly evident, emphasizing their role in transforming patient involvement and compliance in trials. Moreover, organizations like Quanticate’s Clinical Data Management team are prioritizing the incorporation of technology into trials for precise, regulatory-compliant data collection, reflecting current industry practices and trends.
Navigating Regulatory Compliance for Wearable Devices
Navigating the regulatory landscape for smart devices in trials necessitates a comprehensive understanding of the guidelines established by regulatory bodies such as the FDA and EMA. Researchers must ensure that the devices employed meet stringent safety and efficacy standards, often requiring the appropriate approvals prior to deployment in medical settings. As we approach 2025, the emphasis on compliance with privacy regulations, including HIPAA, remains paramount, particularly as wearables frequently collect sensitive health information from participants.
To facilitate adherence to these regulations, researchers should conduct thorough assessments of wearable devices, ensuring they are validated for clinical use and that management practices are robust and compliant with current standards. This proactive approach not only safeguards participant information but also bolsters the credibility and integrity of the research. bioaccess® is dedicated to ensuring information security and client trust, with a dedicated Grievance Officer available to address any concerns regarding data processing, thus ensuring compliance and transparency in all operations.
For any questions or issues, clients can reach out to our Grievance Officer at info@bioaccessla.com.
Additionally, recent statistics indicate a rising trend in FDA and EMA approvals for wearable medical devices, reflecting a growing acknowledgment of their potential in medical research. For instance, the number of FDA approvals for technology devices has risen significantly over the past few years, highlighting the evolving landscape of regulatory acceptance. Successful case studies demonstrate that wearable device clinical trial design requires adherence to regulatory guidelines, such as those specified by the MHRA in the UK and the FDA in the US regarding safety, performance, and information integrity, which are vital for the effective incorporation of devices into trials.
Moreover, bioaccess® provides extensive services that encompass feasibility evaluations, investigator selection, regulatory compliance assistance, and project management, all essential for overcoming the obstacles faced by medical device startups in trials, including regulatory challenges and recruitment concerns.
By emphasizing regulatory adherence and engaging in ongoing education, researchers can enhance the design of wearable device clinical trials, thereby ensuring both participant safety and the reliability of the information gathered.
Best Practices for Selecting Wearable Devices
Choosing the appropriate wearable device clinical trial design necessitates a thorough assessment of essential factors: functionality, user-friendliness, and accuracy. Devices must capture pertinent health metrics that align with the project's objectives, ensuring that the data collected is both relevant and actionable. In wearable device clinical trial design, user-friendliness is particularly crucial; participants are more likely to adhere to study protocols when the devices are comfortable and intuitive to use.
Research indicates that wearables with user-friendly interfaces not only enhance participant satisfaction but also significantly improve compliance rates. Furthermore, the reliability of the information gathered is paramount. Researchers should ensure that the devices have undergone rigorous validation for medical use, as this directly impacts the integrity of the findings. A comparative analysis of different portable devices emphasizes that those featuring strong information-gathering abilities and accessible designs lead to greater participant satisfaction and compliance with protocols.
This highlights the importance of a wearable device clinical trial design that fulfills the technical criteria of the research while focusing on the user experience, ultimately resulting in more successful clinical outcomes. Moreover, representativity in portable technology is essential to guarantee diverse population health information and prevent biased datasets. Combining device-generated data with other health information is crucial for efficient health monitoring and research, as emphasized in a case analysis on interoperability of device data. This integration can address issues of overestimation and enhance the overall effectiveness of healthcare solutions.
As Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, states, "Choosing user-friendly and dependable wearable device clinical trial design is crucial for ensuring participant engagement and maintaining the integrity of trial outcomes."
At bioaccess®, we provide extensive trial management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Trial setup
- Import permits
- Project management
- Reporting
Our expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies ensures that your trials are conducted efficiently and effectively, leveraging over 20 years of experience in the Medtech field. We prioritize the incorporation of portable technology into our studies, ensuring that the devices chosen not only meet regulatory standards but also enhance participant engagement and information integrity.
Data Collection and Management: Leveraging Wearable Technology
To ensure the success of clinical trials, effective management of the vast amounts of information produced by wearable devices is crucial. Implementing robust information management systems capable of handling real-time streaming from wearables is essential. This necessitates establishing comprehensive protocols for data storage, analysis, and reporting, which are critical for maintaining the integrity of the data collected.
At bioaccess®, we leverage over 20 years of expertise in managing trials, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
Our comprehensive trial management services ensure that all aspects of the study, from feasibility assessments to compliance reviews and project management, are meticulously handled.
Prioritizing information quality is equally important; researchers should regularly calibrate devices and conduct validation checks to ensure accuracy. For instance, a research study utilizing a centralized information management platform for wearable information reported substantial enhancements in accuracy and a decrease in entry mistakes. This not only led to more reliable study outcomes but also enhanced the overall efficiency of the trial process.
Furthermore, statistics show that 73% of pharmaceutical firms now utilize cloud-based management systems for trials, an increase from only 45% five years prior, underscoring a notable trend in the sector. The incorporation of technology to handle ongoing information gathered by devices, in contrast to conventional timed information gathering at medical facilities, is a revolutionary advancement. By employing effective information management strategies, researchers can utilize the vast physiological and activity information collected from devices within the framework of wearable device clinical trial design to make more informed health decisions.
This capability is crucial for understanding patient health and activity levels, ultimately improving patient care.
Moreover, sponsors will continue to employ proof of concepts (POC) to assess and confirm the practicality of devices in research, further highlighting the necessity for efficient information management. A case examination titled "Information Gathering and Analysis in Smart Technology" demonstrates that the capacity to assess long-term information collected from such devices improves the precision of health evaluations and enables more informed medical choices, ultimately enhancing patient care.
Additionally, statistics indicate that pharmaceutical companies implementing end-to-end information management solutions can reduce their drug development cycle by an average of 1.5 years and increase their likelihood of regulatory approval by 23%. Such insights emphasize the essential role of efficient information management in enhancing trial results, especially regarding wearable technology, and underscore the importance of collaborating with bioaccess® for your research requirements.
Challenges and Considerations in Wearable Device Trials
The design of wearable device clinical trials presents a wealth of opportunities for medical research, yet it also introduces a range of challenges that must be navigated effectively. Key technical issues, including connectivity disruptions and battery life limitations, can significantly hinder information collection and participant adherence. Moreover, participants may experience discomfort or confusion when adapting to new technologies, adversely affecting their engagement levels.
To address these challenges, it is crucial for researchers to implement comprehensive training programs tailored to participants. Such training should focus on familiarizing users with the devices, ensuring they are comfortable and confident in their usage. Evidence indicates that effective participant training in wearable device clinical trial design can enhance compliance and data quality, ultimately leading to more reliable outcomes in clinical trials.
Furthermore, conducting pilot tests is an invaluable strategy for identifying potential obstacles prior to the full-scale trial. These preliminary studies allow researchers to test the wearable technology in a controlled environment, enabling them to make necessary adjustments based on real-world feedback. This proactive approach not only enhances the design of the wearable device clinical trial but also fosters a smoother experience for participants.
A recent analysis titled 'Factors Influencing Quality in Wearable Devices' identified three intrinsic quality challenges: completeness, correctness, and heterogeneity. Addressing these challenges is essential for ensuring high-quality information collection. Retaining raw sensor information can facilitate the adoption of improved health metrics while maintaining compliance with regulatory integrity requirements.
As Christine Guo, PhD, pointed out, "With their ability for real-time, objective information gathering and continuous oversight, these devices act as channels that effortlessly capture patients’ experiences, free from the recall biases that can affect traditional methods." By emphasizing participant training and pilot studies, along with the preservation of unprocessed sensor information, researchers can enhance the utilization of portable devices, ultimately advancing the field of medical research.
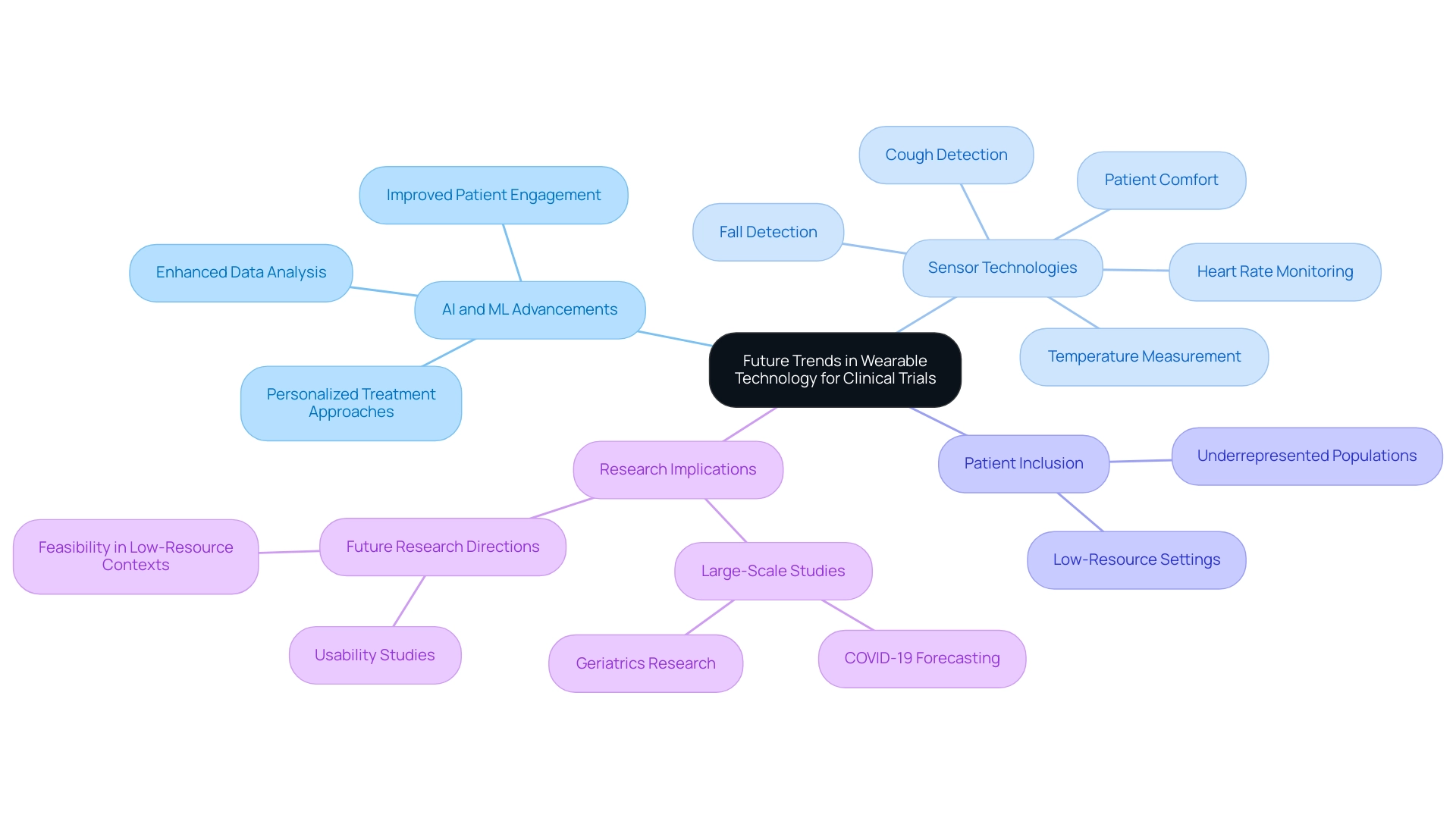
Future Trends in Wearable Technology for Clinical Trials
The future of personal technology in wearable device clinical trial design is exceptionally promising, driven by rapid advancements in artificial intelligence (AI) and machine learning (ML) that are set to revolutionize data analysis and patient monitoring. Emerging trends highlight the development of sophisticated sensors capable of capturing a broader spectrum of health metrics, alongside the seamless integration of devices with telehealth platforms for enhanced remote patient monitoring.
Recent innovations in personal technology have introduced sensors that monitor heart rate, detect coughs, measure temperature, and identify falls, all while prioritizing patient comfort. These advancements not only enhance data gathering but also promote the inclusion of underrepresented populations in research, addressing a critical gap in clinical investigations. A significant research project titled "Wearables in Health Research" underscores the growing use of fitness trackers in large-scale, population-based investigations, particularly in areas like COVID-19 forecasting and geriatrics.
This research emphasizes the potential of devices to involve underrepresented groups, while also urging for future investigations to examine their usability in low-resource environments. Furthermore, a study conducted in North America in 2019 involved over 8 million participants, underscoring the potential scale of wearable device applications in health research.
Researchers must remain vigilant about these developments, as they can profoundly influence the design and execution of wearable device clinical trials. AI-driven analytics can yield deeper insights into patient information, enabling more personalized treatment approaches and enhancing overall trial efficiency. As Christine Guo, PhD, highlights, sponsors aiming to integrate wearable-derived measures into their healthcare programs should thoroughly assess factors such as measurement accuracy, patient comfort, and the specific health metrics being monitored to maximize the advantages of these technologies.
Looking forward, the incorporation of AI and ML in device research is anticipated to grow, with forecasts suggesting a notable rise in the application of these technologies for trials. This trend not only promises to enhance data analysis but also to improve patient engagement and compliance, ultimately leading to more successful health outcomes. As the landscape of wearable technology continues to evolve, it is crucial for clinical researchers to adapt their strategies in wearable device clinical trial design to leverage these innovations effectively.
Conclusion
The integration of wearable devices in clinical trials signifies a pivotal evolution in healthcare research, presenting numerous advantages that enhance data collection, patient engagement, and overall trial efficiency. These devices facilitate real-time monitoring of health metrics, which not only boosts data accuracy but also encourages greater participant adherence to study protocols. This shift towards the utilization of wearables is underscored by the growing acknowledgment of their potential, as evidenced by recent statistics indicating an increase in FDA approvals for such technologies.
While the benefits are apparent, researchers must also confront challenges related to regulatory compliance, data management, and participant training. Adhering to rigorous safety and efficacy guidelines is paramount, as is ensuring that the selected wearable devices are user-friendly and capable of capturing pertinent health data. Comprehensive training programs and pilot studies can alleviate common issues associated with technology adoption, ultimately enhancing participant comfort and data quality.
Looking ahead, the future of wearable technology in clinical trials appears promising, propelled by advancements in artificial intelligence and machine learning. These innovations are poised to revolutionize data analysis and patient monitoring, enabling the capture of a broader spectrum of health metrics and the inclusion of diverse populations in research endeavors. As the landscape continues to evolve, researchers must remain proactive in adapting their strategies to effectively leverage these technologies, ensuring that clinical trials not only yield reliable data but also contribute to improved patient outcomes across the board. The potential of wearables to transform clinical research is substantial, and embracing these tools is essential for advancing healthcare innovation.
Frequently Asked Questions
How are wearable devices transforming clinical research trials?
Wearable devices, such as smartwatches and fitness trackers, facilitate continuous monitoring of participants' health metrics, capturing vital signs and physical activity in real-time. This provides researchers with a comprehensive dataset, enhances information gathering, and promotes improved patient outcomes.
What percentage of trials focus on the precision and reliability of wearable devices?
Recent statistics indicate that 58.1% of trials emphasize the precision and dependability of wearable devices, highlighting their increasing significance in clinical research.
How do wearable devices impact trial management efficiency?
The automation of data capture from wearable devices significantly reduces the administrative burden on research teams, enhancing overall trial management efficiency.
What benefits do participants receive from wearable devices during trials?
Participants benefit from real-time monitoring of health metrics, which may include reminders for medication or appointments, leading to improved engagement and adherence to study protocols.
Why is it important to enhance wearable technology for varied populations?
Enhancing wearable technology for usability across varied populations, particularly in low-resource settings, is crucial for bridging gaps in information collection and participant representation in health research.
What concerns are associated with the algorithms used in personal technology for research?
The effects of undisclosed algorithms can significantly influence information analysis and standardization, emphasizing the necessity for transparency in personal technology.
How is the quality of research utilizing personal devices evaluated?
Case analyses have established a framework for assessing the reliability of research results, using instruments like the Medical Education Research Study Quality Instrument to ensure credible research designs.
What role do wearable devices play in enhancing patient engagement in clinical trials?
Wearable devices provide immediate feedback on health metrics, which sustains participant motivation and adherence to study protocols, leading to improved engagement and retention rates.
What impact does immediate health feedback have on patient compliance?
Studies suggest that immediate health feedback can lead to higher compliance rates, with reports indicating that patient adherence can increase by as much as 30% when utilizing wearable technology.
What challenges are faced regarding the regulation of wearable devices in clinical research?
The regulation of wearable devices varies, with many consumer products lacking published accuracy information, highlighting the need for a multidisciplinary approach to address these challenges in oncology care.
How does wearable technology influence patient involvement in trials?
The prompt feedback provided by wearable devices enhances patient involvement and compliance in trials, reflecting their potential to transform the landscape of medical research.




