Overview
The article emphasizes best practices in clinical study design for implants, underscoring the critical need for a clear framework, methodological rigor, and regulatory compliance to secure successful outcomes. It articulates strategies such as:
- Randomization
- Blinding
- Effective patient recruitment
While asserting the necessity for robust data management and adherence to regulatory standards. These elements are essential to enhance the credibility and effectiveness of medical device trials, ultimately fostering trust in clinical research.
Introduction
In the rapidly evolving field of medical technology, the design of clinical studies for implants is pivotal in advancing patient care and ensuring safety. As researchers strive to establish effective methodologies, they must navigate a complex landscape of regulatory requirements, recruitment challenges, and data management issues.
From formulating precise research questions to implementing robust data collection protocols, every aspect of study design is critical for yielding credible results. This article delves into the fundamental principles of clinical study design, exploring best practices, regulatory considerations, and innovative strategies that enhance the effectiveness of implant studies.
By understanding these elements, stakeholders can contribute to the successful development of medical devices, ultimately leading to improved patient outcomes and fostering growth within the Medtech sector.
Fundamentals of Clinical Study Design for Implants
Successful clinical study design for implants begins with a clear expression of the inquiry and goals. Establishing a robust framework is essential, encompassing the research type—such as randomized controlled experiments or observational analyses—along with defining population characteristics and the specific technology being assessed. Key elements like randomization and blinding are crucial to minimize bias and enhance the validity of results.
Indeed, recent statistics demonstrate that research utilizing these methodologies produces more dependable outcomes, underscoring their significance in clinical study design for implants.
Moreover, the research protocol must meticulously outline methods for data collection, monitoring, and analysis, ensuring adherence to regulatory standards and ethical guidelines. bioaccess® strictly adheres to these standards, facilitating the gathering of high-quality data while bolstering the credibility of the findings. This, in turn, contributes to enhanced patient outcomes and promotes advancements in device technology.
With over 20 years of experience in the Medtech industry, bioaccess® is ideally positioned to assist researchers in applying these best practices, particularly through its comprehensive trial management services, which include:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
Recent trends in clinical study design for implants reveal a shift towards insourced data management models, where sponsors take greater control over their data processes. This movement is driven by the demand for data transparency and operational efficiency, allowing for improved research quality and better patient outcomes. A notable case analysis titled "Shifting Towards Insourced Data Management Models" illustrates this trend, highlighting how sponsors are moving away from reliance on CROs to ensure superior data management and outcomes.
Additionally, challenges in scaling metadata management persist, as many companies still rely on spreadsheets despite the shift to electronic data capture (EDC). Addressing these obstacles is essential for enhancing the overall effectiveness of medical research.
As Vivienne van der Walle, Founder and Medical Director, aptly notes, "Anything that takes away time from patients is a pain point for a site, and anyone who resolves that is helping patient care." This underscores the significance of efficiency in research and its direct effect on patient care.
In summary, the incorporation of optimal methods in clinical study design for implants, including the strategic use of randomization and blinding, is crucial for the success of medical device trials. By focusing on these aspects, researchers can ensure that their investigations not only fulfill regulatory requirements but also contribute significantly to the advancement of medical technology, particularly regarding bioaccess®'s expedited medical device evaluation services in Latin America. Furthermore, the successful implementation of these analyses positively impacts local economies, fostering job creation and economic growth in the region.
Regulatory Considerations in Implant Clinical Studies
Regulatory factors play a vital role in the clinical study design for implants, necessitating researchers to skillfully navigate a complex environment influenced by directives from major regulatory organizations like the FDA and EMA. A fundamental aspect of this process is obtaining Investigational Device Exemption (IDE) approval, essential for conducting studies involving significant risk devices. The approval statistics for IDE applications in 2025 indicate a growing trend, reflecting the increasing recognition of the importance of innovative device technologies in patient care.
Moreover, it is crucial to note that clinical trials must be registered in publicly available databases, such as ClinicalTrials.gov in the US and EudraCT in the EU. This regulatory requirement ensures transparency and adherence, reinforcing the integrity of the research process.
Compliance with Good Clinical Practice (GCP) is another cornerstone of successful clinical study design for implants. Researchers must ensure that their investigations adhere to rigorous ethical and scientific standards, which not only facilitates regulatory approval but also enhances the credibility of the research findings. Engaging with regulatory authorities early in the research design process is essential; this proactive approach allows for clarification of expectations and can significantly streamline the approval process.
Incorporating insights from FDA and EMA officials regarding device research regulations can further enhance the design process. For instance, the introduction of Parallel Scientific Advice (PSA) by the EMA and FDA facilitates concurrent discussions on scientific issues, potentially reconciling differing regulatory perspectives. Although participation in PSA is not guaranteed and may be limited based on specific criteria, it represents a valuable opportunity for researchers to align their research designs with regulatory expectations.
Furthermore, understanding the specific reporting requirements set forth by the FDA and EMA is vital. This includes the necessity for post-market surveillance and the timely reporting of adverse events, integral to maintaining compliance and ensuring ongoing patient safety. By incorporating these regulatory factors into the clinical study design for implants, investigators can effectively reduce risks and enhance the probability of achieving successful results in their evaluations of devices.
Ultimately, a comprehensive understanding of regulatory guidelines for medical examinations on devices, along with a strategic approach to clinical study design for implants, IDE approval, and compliance, is crucial for advancing medical products in the Medtech sector. bioaccess®'s comprehensive management services for research encompass feasibility assessments, site selection, compliance reviews, setup processes, import permits, project oversight, and reporting of serious and non-serious adverse events, all aimed at supporting the successful navigation of the regulatory landscape and contributing to the overall advancement of innovative medical technologies. To learn more about how we can assist you, BOOK A MEETING.
Methodologies for Effective Clinical Study Design
Efficient clinical study design for implants necessitates a variety of research methodologies tailored to specific research goals. Randomized controlled studies (RCTs) are widely acknowledged as the gold standard in clinical research, particularly within the framework of clinical study design for implants, owing to their capacity to minimize bias and facilitate robust comparisons between treatment groups. Recent statistics reveal that 76.2% of adaptive RCTs conducted were post-market trials, underscoring the relevance of RCTs in assessing medical devices after they have entered the market.
While RCTs are often favored, observational research can also be suitable, especially in situations where RCTs may be impractical due to ethical or logistical constraints. Researchers must meticulously consider the statistical analyses employed, ensuring they are appropriately aligned with the data type and research design. This consideration is critical for maintaining the integrity of the findings.
In recent years, adaptive study designs have gained momentum in clinical study design for implants, providing the flexibility to modify study parameters in response to interim results. An analysis of 105 medical device RCTs with adaptive designs indicated a total of 2.112 adaptive RCTs per 1000 medical device clinical studies registered from 2000 to 2024. However, the findings also revealed a decline in the average annual number of adaptive RCTs over the past eight years, attributed to challenges such as limited study information and concerns regarding data integrity and ethics.
As highlighted in the case study titled "Adaptive Designs in Medical Device Clinical Trials," this decline underscores the complexities encountered in effectively implementing adaptive designs.
According to the NMPA, "Adaptive design refers to a type of design in which modifications are made to one or more aspects of a study during the process, utilizing accumulated data that are preplanned in the protocol." This definition clarifies the concept of adaptive design and accentuates its significance in trial methodologies.
Furthermore, large simple RCTs can mitigate the risk of overinterpreting the effects of medical devices by increasing the number of participating centers, thereby offering a broader perspective on RCT methodologies and their significance in research.
Ultimately, the methodologies selected in the clinical study design for implants must align with the objectives and regulatory requirements, ensuring that the findings are both scientifically sound and clinically relevant. By prioritizing patient autonomy, researchers should guarantee that patients are fully informed about the risks and benefits associated with device use, empowering them to make informed decisions. This comprehensive approach to research design not only enhances the quality of investigations but also contributes to the advancement of medical devices that can significantly improve patient outcomes.
At bioaccess®, we leverage our 20+ years of expertise in managing research projects, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up assessments, along with comprehensive services such as compliance reviews, setup, and project management, to ensure that your medical investigations are conducted with the highest standards of quality and adherence in Latin America. Our commitment to regulatory excellence further enhances our capability to navigate the complexities of trials effectively.
Patient Selection and Recruitment Strategies
Efficient patient selection and recruitment approaches are essential for the success of clinical study design involving implants. Researchers must establish precise inclusion and exclusion criteria tailored to the study's objectives and the specific characteristics of the implant under investigation. Engaging with patient advocacy groups proves particularly beneficial; these organizations can mobilize support and enhance outreach efforts, leading to a more diverse participant pool.
Engaged patients are more likely to complete trials and become advocates for future medical research, further emphasizing the value of these groups.
In Barranquilla, Colombia, the collaboration between bioaccess™ and Caribbean Health Group aims to position the city as a leading destination for trials in Latin America, supported by Colombia's Minister of Health. This initiative not only improves the recruitment landscape but also cultivates a supportive environment for research. Utilizing social media platforms amplifies recruitment initiatives, allowing researchers to connect with potential participants in innovative ways.
Clear communication regarding the project's purpose, procedures, and potential benefits is essential in alleviating patient concerns and fostering trust. This transparency encourages participation and can significantly improve enrollment rates. Furthermore, strategies such as referral programs and community outreach initiatives can strengthen recruitment efforts, engaging local networks and enhancing awareness of the study.
Consistent monitoring and assessment of recruitment objectives are vital for trial success, ensuring that strategies remain effective and adjustable to the requirements of various trials. By prioritizing patient-centric approaches and actively involving advocacy groups, researchers can enhance recruitment while ensuring that the participant population accurately reflects the broader patient demographic. This alignment with best practices ultimately contributes to the robustness and credibility of clinical study design for implants in the implant sector.
Moreover, bioaccess®'s expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies underscores the significance of thorough research management services. As noted by SubjectWell, innovative solutions like their OneView recruitment portal can transform patient recruitment, showcasing the potential for technology to streamline these efforts. Furthermore, the partnership with GlobalCare Clinical Trials has demonstrated notable advancements in recruitment efficiency, achieving over a 50% decrease in recruitment duration and 95% retention rates, emphasizing the impact of strategic collaborations in improving research outcomes.
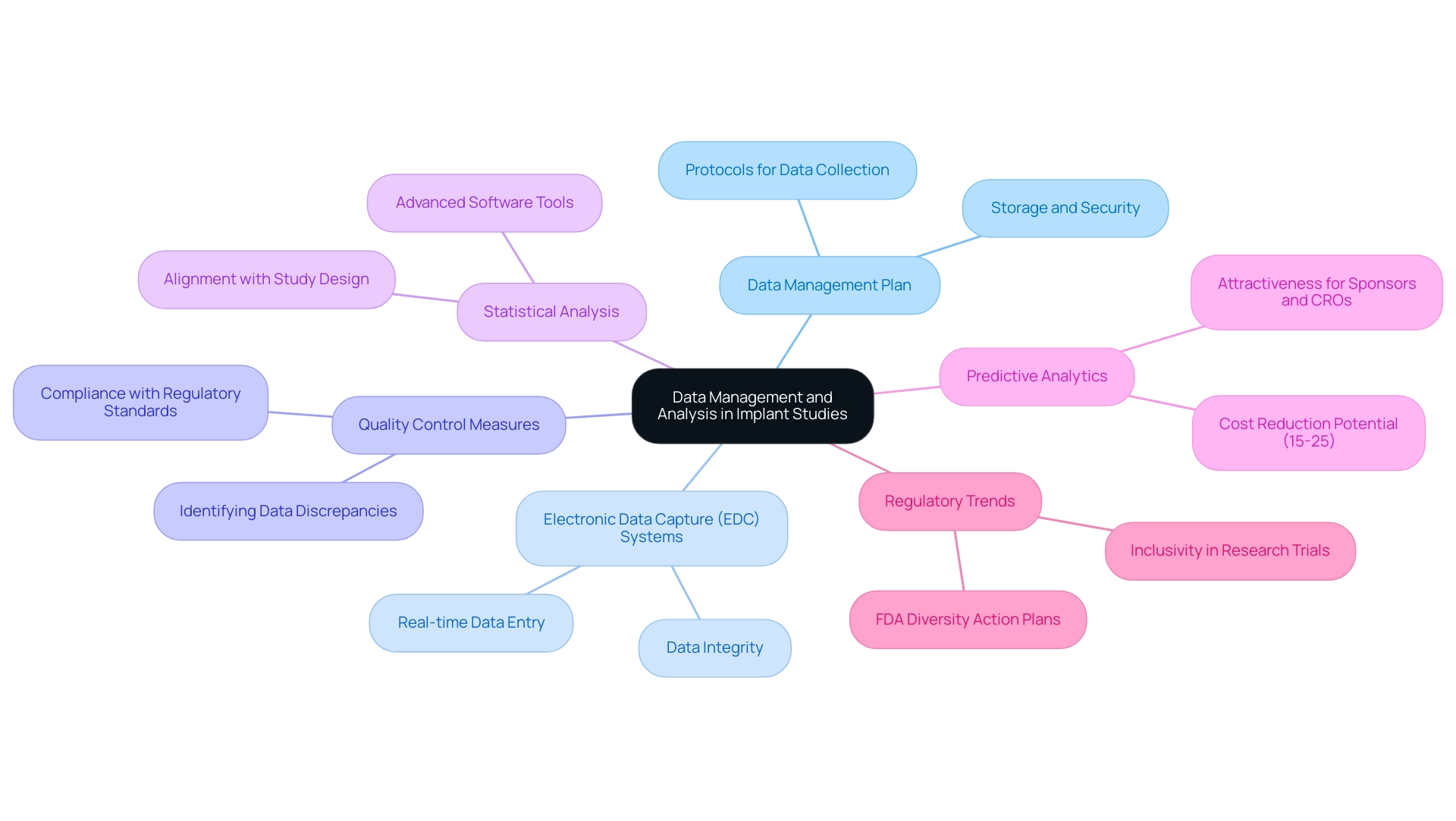
Data Management and Analysis in Implant Studies
Effective data management and analysis are paramount for the success of trials involving implants, particularly within the framework of clinical study design for expedited medical device research services in Latin America provided by bioaccess®. Researchers must develop a robust data management plan that articulates protocols for data collection, storage, and security. The adoption of electronic data capture (EDC) systems is essential, as these technologies significantly enhance data accuracy and streamline the overall management process.
In 2025, the emphasis on EDC systems is underscored by their capacity to facilitate real-time data entry and monitoring, which is crucial for maintaining data integrity. Implementing stringent quality control measures is vital to swiftly identify and address any data discrepancies. This proactive approach not only safeguards the reliability of the data but also ensures compliance with regulatory standards, aligning with bioaccess®'s commitment to regulatory excellence in clinical trials. Statistical analysis should be performed using advanced software tools, with careful consideration given to the alignment of chosen methods with the study's design and objectives.
By prioritizing data integrity and employing rigorous analytical techniques, researchers can generate trustworthy results that significantly contribute to the evolving landscape of implant technology. Furthermore, recent insights suggest that predictive analytics could lower trial expenses by 15-25%, making it an attractive approach for sponsors and contract research organizations (CROs). As Dhruvesh Patel noted, with real-world evidence (RWE), blockchain, and digital platforms at the forefront, 2025 promises a research environment that is faster, more secure, and more aligned with the needs of patients and stakeholders alike. Additionally, the case analysis titled "Enhancing Site Experience in Clinical Trials" illustrates how innovations in data management can simplify site operations and improve patient care, demonstrating the practical benefits of these strategies.
Moreover, the new FDA draft guidance on Diversity Action Plans emphasizes the importance of inclusivity in research trials, aligning with current regulatory trends. As the research environment evolves, integrating innovative data management practices will be crucial for optimizing the efficiency and effectiveness of trials in the Medtech sector, particularly as bioaccess® continues to lead in this field.
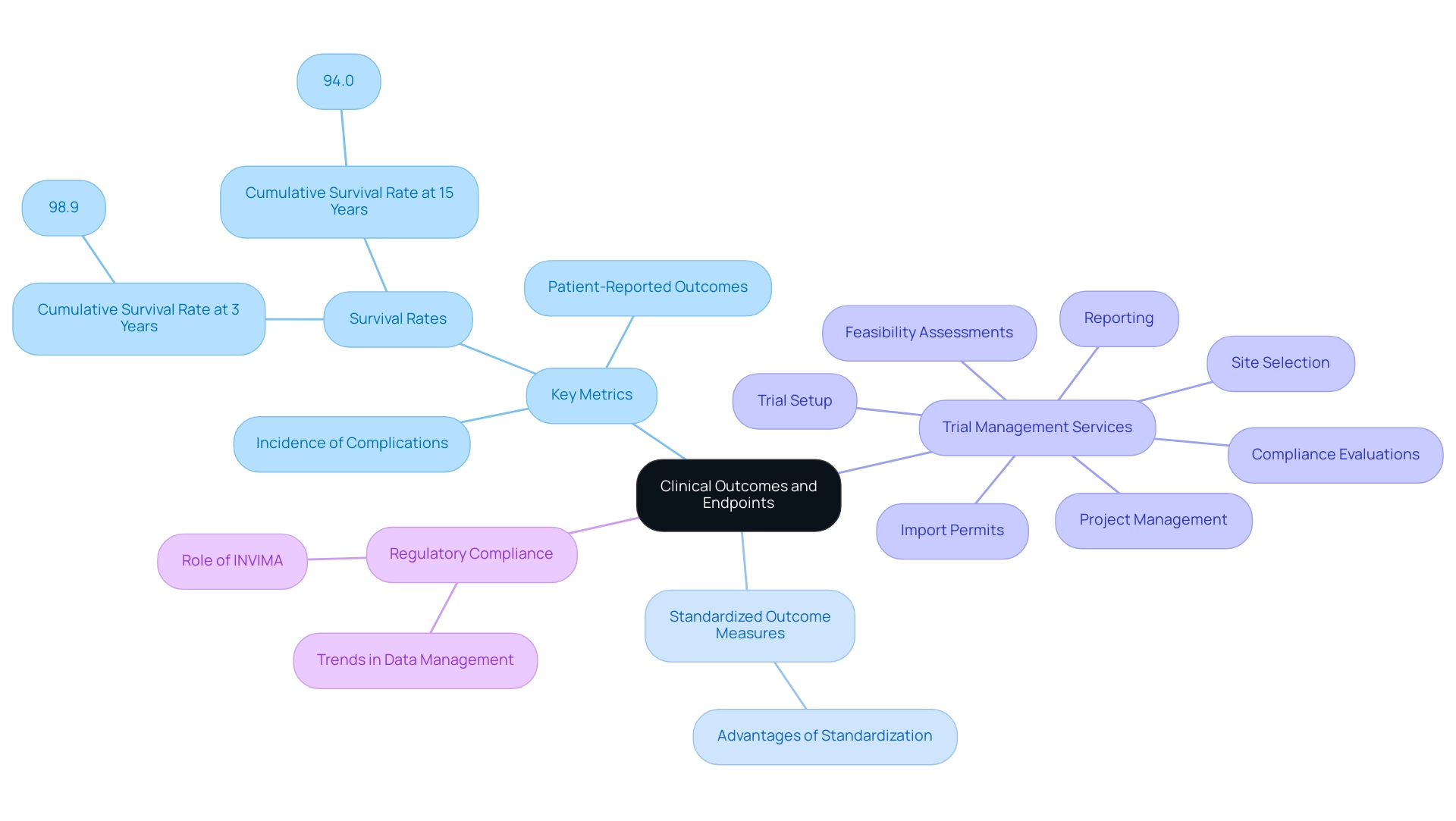
Defining Clinical Outcomes and Endpoints
Defining clinical outcomes and endpoints is a crucial element of clinical study design for implants, directly impacting the assessment of a device's effectiveness and safety. Researchers must carefully identify outcomes that align with the project's objectives, including critical metrics such as:
- Survival rates of devices
- Patient-reported outcomes
- Incidence of complications
These endpoints should not only be measurable and clinically meaningful but also adhere to regulatory standards to ensure compliance and credibility.
The adoption of standardized outcome measures is especially advantageous, enabling consistent comparisons across various research, thereby enhancing the robustness of the findings. For instance, in a longitudinal observational cohort study involving 10,871 dental devices, researchers documented a cumulative survival rate of 98.9% at three years and 94.0% at fifteen years, underscoring the importance of clear metrics in assessing long-term performance. Furthermore, the average marginal bone level was noted at 0.09 ± 0.28 mm during stage two surgery and 0.49 ± 0.74 mm after 8–10 years, emphasizing the importance of measurable results in assessing success.
Moreover, it is essential for researchers to consider both short-term and long-term outcomes in the clinical study design for implants to provide a comprehensive evaluation of the device's performance. This dual focus enables a thorough understanding of how implants perform over time and their overall impact on patient health. As Max Baumann, Head of Execution at Treehill Partners, pointed out, "Entering 2025, we still observe biotech encountering essential business model difficulties as end-markets become increasingly crowded."
This underscores the necessity for clear and relevant endpoints in the competitive arena of medical research. In this context, bioaccess provides extensive trial management services that encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Trial setup
- Import permits
- Project management
- Reporting
By establishing clear and relevant endpoints within the clinical study design for implants, researchers can effectively gauge the success of the implant, ultimately contributing to improved patient outcomes and advancing the field of medical technology. Moreover, with a rising trend for sponsors to centralize data management internally, there is a heightened focus on ownership and clarity of data, which can affect research design and the definition of health outcomes.
Additionally, understanding the role of INVIMA, the Colombia National Food and Drug Surveillance Institute, is crucial as it oversees regulatory functions related to medical devices, ensuring compliance with health standards. The influence of Medtech clinical research extends beyond clinical outcomes; they also contribute to local economies through job creation, economic growth, and healthcare enhancement, fostering international collaboration in the field.
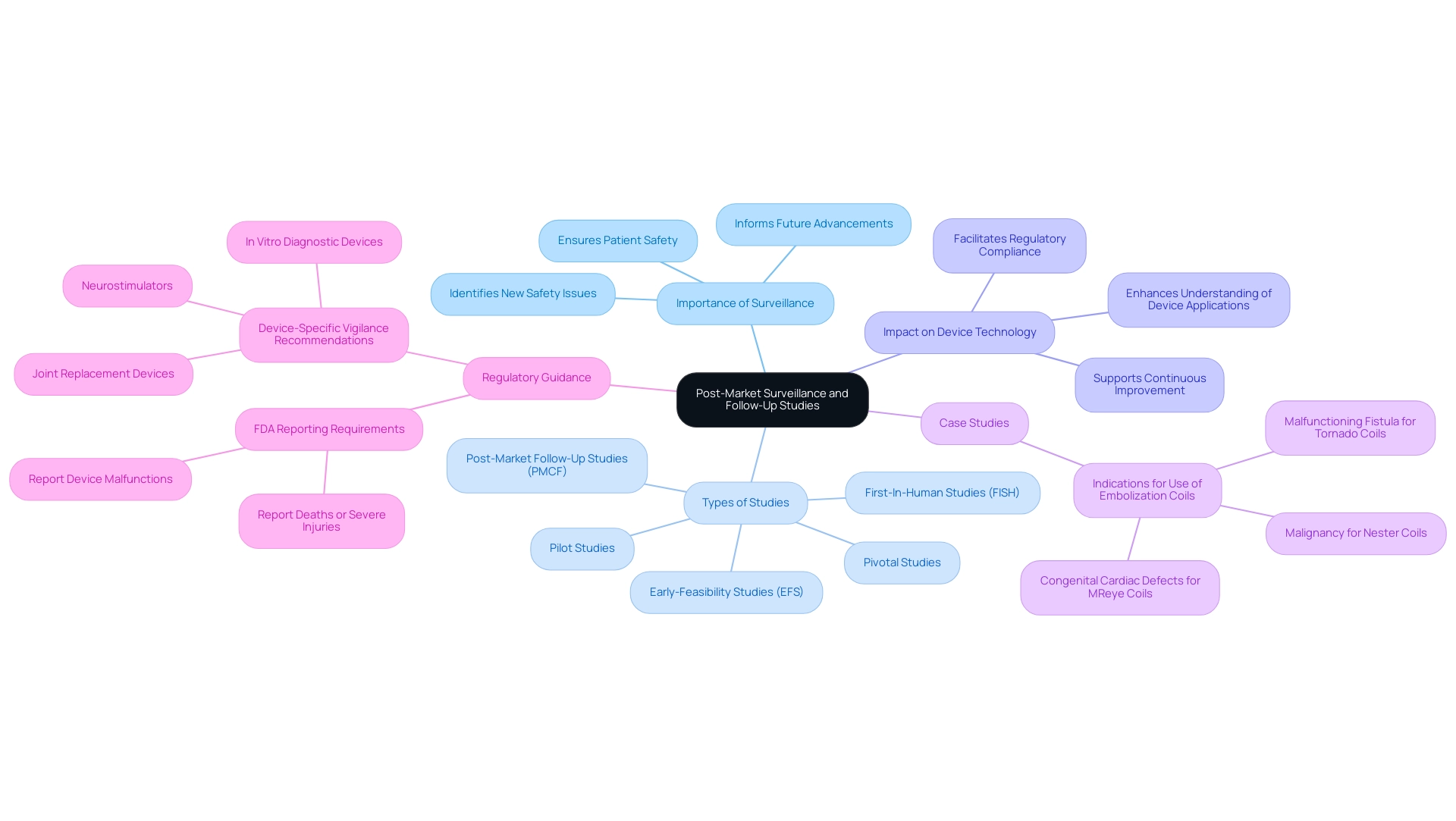
Post-Market Surveillance and Follow-Up Studies
Post-market monitoring and follow-up research are critical components in ensuring the safety and effectiveness of medical devices post-commercialization. A well-structured post-market surveillance plan must encompass systematic monitoring of patient outcomes, adverse events, and device performance metrics. Engaging healthcare providers and patients is essential for gathering meaningful data on long-term outcomes, which can significantly inform future advancements in implant technology.
At bioaccess®, we leverage over 20 years of expertise in managing studies to ensure that post-market follow-up research is conducted with the highest standards of innovation and regulatory excellence. Our tailored approach emphasizes:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FISH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
This positions us as leaders in Medtech research throughout Latin America. We also guide companies through the complexities of trials, facilitating successful acquisition outcomes.
Recent research has highlighted the importance of follow-up investigations in evaluating the longevity and efficacy of devices over time. These investigations not only help identify new safety issues but also provide essential insights into the medical indications for various device types. A notable case analysis on embolization coils illustrated how automated data mining can uncover specific indications for use, such as malignancy for Nester Coils and congenital cardiac defects for MReye Coils, thereby enhancing our understanding of their application in clinical settings.
This automated data extraction process underscores the practical utility of follow-up research in advancing device technologies.
As we progress into 2025, the emphasis on post-market monitoring strategies has intensified, with new device-specific vigilance recommendations issued for a range of medical devices, including joint replacement devices and neurostimulators. This guidance reinforces the necessity for manufacturers to report any incidents that may lead to severe injury or malfunction, as stated by the FDA: "Manufacturers are required to report to the FDA when they learn that any of their devices may have caused or contributed to a death or severe injury."
Statistics reveal that over 600 hours of manual labor were necessary to compile and analyze data related to post-market surveillance, highlighting the resource-intensive nature of these efforts. Statistical analyses were performed using SAS software, reporting continuous variables as mean ± standard deviation and categorical variables as percentages and frequencies. However, the investment in such thorough follow-up research is justified by the potential to enhance patient safety and foster ongoing advancements in device technologies.
By prioritizing these practices, researchers can ensure that the benefits of medical devices are maximized while minimizing risks to patients.
Challenges and Solutions in Clinical Study Design
Clinical study design for implants presents numerous challenges, including recruitment difficulties, regulatory hurdles, and data management issues. Recruitment remains a significant barrier, with research indicating that nearly 80% of studies fail to meet their enrollment targets. To address this, researchers should implement targeted outreach strategies, such as collaborating with patient advocacy groups and leveraging social media platforms to effectively engage potential participants. This strategy not only expands the recruitment pool but also builds trust within patient communities.
In Colombia, the partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a leading hub for clinical research in Latin America, with the support of Colombia's Minister of Health. This initiative is anticipated to bolster recruitment efforts and streamline processes, ultimately enhancing trial outcomes.
Regulatory hurdles represent another critical concern, particularly as regulations become increasingly intricate and prescriptive. As noted by Dipanwita Das, CEO & co-founder, "Last, but certainly not the least is regulatory preparedness. Regulations are getting more complex and more prescriptive and more demanding..." Maintaining open lines of communication with regulatory authorities, such as INVIMA, Colombia's National Food and Drug Surveillance Institute, is vital for navigating these challenges. By staying informed about evolving guidelines and incorporating feedback from regulatory bodies throughout the research process, researchers can ensure compliance and facilitate a smoother path to commercialization.
Data management challenges can be effectively mitigated through the implementation of advanced technologies, such as electronic data capture systems, which streamline data collection and enhance accuracy. Additionally, rigorous quality control measures should be instituted to safeguard data integrity. A case analysis titled 'Challenges in Clinical Trial Design' underscores the necessity for innovative clinical study design for implants that addresses real-world implementation challenges. This shift is expected to yield more meaningful and translatable research outcomes across diverse global settings.
Furthermore, adopting risk-based approaches can lead to higher data quality, greater resource efficiency, and shorter research timelines. By proactively identifying and addressing these challenges, researchers can significantly enhance the efficiency and success of clinical study design for implants, ultimately contributing to improved patient outcomes and the advancement of medical technology. Additionally, as emphasized by Joseph Tucker, defining and measuring appropriate outcomes in clinical trials is crucial for ensuring the relevance and impact of the research.
Conclusion
Effective clinical study design for implants is crucial for advancing patient care and ensuring the safety and efficacy of medical devices. Key principles such as precise research questions, robust methodology, and compliance with regulatory standards are foundational elements emphasized throughout the article. By employing best practices—including randomization, blinding, and comprehensive data management—researchers can generate credible results that significantly contribute to the Medtech sector.
Navigating the regulatory landscape is essential for successful study execution, particularly in obtaining Investigational Device Exemption (IDE) approvals and adhering to Good Clinical Practice (GCP). The importance of post-market surveillance and follow-up studies cannot be overstated; these efforts provide critical insights into the long-term performance of implants and ensure ongoing patient safety. Furthermore, collaboration with patient advocacy groups and leveraging innovative recruitment strategies are effective methods to enhance participant engagement and streamline the recruitment process.
In conclusion, integrating these methodologies and considerations not only enhances the quality of clinical research but also fosters advancements in implant technology that directly impact patient outcomes. As the field of medical technology continues to evolve, the commitment to rigorous study design and regulatory compliance will be paramount in ensuring the successful development and implementation of innovative medical devices. Through these concerted efforts, stakeholders can drive growth in the Medtech sector while prioritizing patient safety and care.
Frequently Asked Questions
What is the foundation of successful clinical study design for implants?
Successful clinical study design for implants begins with a clear expression of the inquiry and goals, establishing a robust framework that includes the research type, population characteristics, and the specific technology being assessed.
Why are randomization and blinding important in clinical studies?
Randomization and blinding are crucial to minimize bias and enhance the validity of results, leading to more dependable outcomes in clinical research.
What must a research protocol outline in clinical studies?
A research protocol must meticulously outline methods for data collection, monitoring, and analysis, ensuring adherence to regulatory standards and ethical guidelines.
How does bioaccess® contribute to clinical study design?
Bioaccess® adheres to regulatory standards, facilitating the gathering of high-quality data which enhances the credibility of findings and contributes to improved patient outcomes and advancements in device technology.
What services does bioaccess® offer for trial management?
Bioaccess® offers comprehensive trial management services including feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
What recent trends are observed in clinical study design for implants?
There is a shift towards insourced data management models, where sponsors take greater control over their data processes to improve transparency and operational efficiency, leading to better research quality and patient outcomes.
What challenges remain in metadata management for clinical studies?
Many companies still rely on spreadsheets for data management despite the shift to electronic data capture (EDC), which presents challenges in scaling metadata management.
What is the significance of efficiency in clinical research?
Enhancing efficiency in research directly affects patient care by minimizing time taken away from patients, thereby improving overall patient outcomes.
What role do regulatory factors play in clinical study design for implants?
Regulatory factors are vital, requiring researchers to navigate a complex environment influenced by directives from organizations like the FDA and EMA, including obtaining Investigational Device Exemption (IDE) approval for high-risk devices.
Why is it important for clinical trials to be registered in publicly available databases?
Registration in databases like ClinicalTrials.gov and EudraCT ensures transparency and adherence to regulatory requirements, reinforcing the integrity of the research process.
What is Good Clinical Practice (GCP) and why is it important?
Good Clinical Practice (GCP) is a set of ethical and scientific quality standards that researchers must adhere to, facilitating regulatory approval and enhancing the credibility of research findings.
How can researchers engage with regulatory authorities effectively?
Engaging with regulatory authorities early in the research design process allows for clarification of expectations and can significantly streamline the approval process.
What is Parallel Scientific Advice (PSA) and its relevance?
Parallel Scientific Advice (PSA) allows for concurrent discussions between FDA and EMA officials on scientific issues, helping researchers align their designs with regulatory expectations.
What are the reporting requirements for clinical studies set by the FDA and EMA?
Researchers must comply with reporting requirements including post-market surveillance and timely reporting of adverse events to maintain compliance and ensure patient safety.

