Overview
Brazil is emerging as a prominent hub for medtech clinical research, driven by its diverse patient demographics, robust regulatory environment, and substantial cost advantages over North America and Europe. Recent investments in healthcare infrastructure, streamlined regulatory processes, and the presence of experienced local organizations like bioaccess® are pivotal in attracting global sponsors and ensuring the success of medical studies. These factors not only enhance the landscape of clinical research but also position Brazil as a strategic choice for international stakeholders seeking to conduct effective and efficient medical studies.
Introduction
Brazil is rapidly emerging as a premier destination for Medtech clinical research, attributed to its strategic location, diverse patient population, and evolving regulatory landscape. As international sponsors increasingly recognize the country's potential, Brazil's commitment to enhancing healthcare and regulatory frameworks is paving the way for successful clinical trials.
Significant investments in healthcare infrastructure and a growing number of clinical studies are transforming the nation into a hub for innovation in medical technology. This article delves into the factors contributing to Brazil's rise, including its demographic advantages, cost efficiency, and the vital role of regulatory bodies like ANVISA, while highlighting the importance of strategic partnerships in navigating this dynamic landscape.
Brazil's Rise as a Leading Medtech Clinical Research Hub
Brazil, strategically located in Latin America, serves as a pivotal hub for medtech clinical research, enabling global sponsors and researchers to access its resources more easily. The nation’s ongoing commitment to enhancing healthcare and regulatory systems significantly boosts its appeal for conducting medical studies, ensuring compliance with international standards. Notably, sponsors are required to pay a Health Surveillance Inspection Fee when submitting a research application, with fees ranging from 983.85 to 19,677 Brazilian Reals, underscoring the regulatory landscape within the country.
Recent investments in healthcare infrastructure are transforming Brazil into a medtech clinical research hub, with funding allocated to modern facilities and technologies that facilitate innovative studies. By 2025, the country is expected to experience a notable increase in the number of medical studies, further solidifying Brazil's position in the medtech arena. This trend reflects the growing confidence of sponsors in the country’s capabilities, particularly with the support of leading CROs like bioaccess®, which specializes in expedited research services for medical devices.
The diverse patient demographic in Brazil presents a vital resource for studies, offering a rich database that enhances the strength and relevance of findings across various populations. As Karla Espirito Santo, leader of Decentralized Studies and New Experimental Models at Hospital Israelita Albert Einstein’s Academic Research Organization, articulates, "The idea is to enhance the diversity of participants in studies, and not just among the small group of patients already visiting centers for investigation."
Ethical considerations are paramount in Brazilian trials, as evidenced by the requirement for approval from an ethics committee when involving participants who are mentally or physically unable to provide consent. This ensures that their rights are respected and that they are adequately informed about the study.
Moreover, ANVISA’s membership in PIC/S and its adherence to globally harmonized GMP inspection standards emphasize Brazil’s commitment to maintaining high regulatory standards in medical studies. Collectively, these factors position Brazil as a medtech clinical research hub, fostering successful international collaborations and driving the advancement of medical technologies. This is further supported by bioaccess®'s expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies. Through bioaccess®, medtech startups can leverage affordable services, including regulatory approval, research site activation, subject recruitment, and data management, ensuring a seamless transition to the next phase of studies and bolstering fundraising efforts.
Navigating Brazil's Regulatory Landscape for Medtech
Acquire a comprehensive grasp of ANVISA's regulations, which are essential for conducting studies in Brazil as a hub for medtech clinical research. These regulations ensure that examinations are carried out ethically and safely, conforming to international standards. Review the recent Law No. 14.874, enacted in May 2024, which significantly simplifies the approval process for medical studies. This law aims to reduce bureaucratic obstacles, thereby accelerating the timeline for introducing innovative medical technologies to the marketplace.
Familiarize yourself with the criteria for ethical approvals, as these can directly influence testing timelines. Understanding the nuances of ethical considerations is crucial for maintaining compliance and ensuring participant safety. For instance, in the United States, children involved in research studies are considered a vulnerable group and require additional protections, underscoring the importance of ethical factors in all medical investigations. Stay informed about ANVISA's regulatory agenda for 2024-2025, which outlines key priorities for Medtech innovations, further establishing Brazil as a hub for medtech clinical research. This agenda reflects the agency's commitment to fostering a supportive environment for medical research and development.
Engage with local regulatory specialists who can provide essential insights into navigating complex compliance challenges. Their expertise can help mitigate risks and enhance the efficiency of the approval process, ensuring that your research studies progress smoothly. Additionally, be aware that the submission of test results may be delayed for up to two years if the sponsor submits a certification indicating that the FDA has not yet authorized the investigational product. This highlights the critical nature of timely approvals in the medical study landscape. Lastly, consider the implications of the FDA's user fees for New Drug Applications, as understanding these costs can offer a broader perspective on the regulatory framework that may influence Brazil as a hub for medtech clinical research. As noted by Aaron S. Kesselheim, adaptive studies play a vital role in regulatory contexts, emphasizing their importance in ensuring compliance. At bioaccess®, we leverage over 20 years of experience in managing research studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). Our extensive research management services ensure that you navigate the complexities of regulatory compliance effectively, enhancing the success of your research initiatives.

Leveraging Brazil's Demographic Advantages for Clinical Trials
Understanding Brazil as a hub for medtech clinical research is essential for successful medical studies. Key elements, including age distribution, ethnic diversity, and prevalent health conditions, significantly influence study outcomes.
Brazil boasts a substantial treatment-naïve population, presenting a unique opportunity for recruiting research participants. This demographic enhances the validity of study results by ensuring that participants have not been previously exposed to treatments that could distort data.
Leveraging local healthcare networks is crucial for accessing diverse patient populations. These networks facilitate connections with potential participants, ensuring that studies accurately reflect the broader Brazilian demographic.
Implementing culturally sensitive recruitment strategies is vital for improving patient engagement and retention. Tailoring communication and outreach efforts to resonate with diverse cultural backgrounds fosters trust and encourages participation.
Analyzing demographic information allows for the personalization of research designs that cater to the specific needs of the Brazilian population. This approach not only enhances the significance of the research but also increases the likelihood of favorable outcomes.
The numerous advantages of conducting studies in Brazil as a medtech clinical research hub include access to a large, treatment-naïve population and a rich demographic tapestry. These factors improve data collection and can lead to more effective medical solutions tailored to the local context.
Furthermore, it is essential to consider socio-economic factors that may affect participant recruitment. Disparities in healthcare access can present ethical challenges, making informed consent particularly complex. Continuous collaboration between sponsors and local investigators is vital to uphold ethical standards and protect vulnerable patient populations.
By leveraging its expertise and tailored strategies, bioaccess® aims to accelerate the development of medical devices efficiently, addressing these challenges through comprehensive research management services. These services encompass feasibility studies, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting. bioaccess® specializes in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and other critical studies, ensuring that research is designed to meet the specific demands of the Brazilian market while navigating the complexities of local regulations and market access challenges.
Cost Efficiency: Why Brazil is a Smart Choice for Medtech Trials
Conducting medical studies in South America, with Brazil positioned as a central hub for medtech clinical research, emerges as a compelling alternative to North America and Europe, particularly when considering financial implications. The medical research market in Brazil is projected to experience significant growth, boasting a compound annual growth rate (CAGR) of 12.85%. This underscores Brazil's status as a medtech research hub and highlights the increasing allure of the region for Medtech firms. A thorough cost analysis reveals that medical studies in South America can be up to 50% less expensive than those in North America and Europe, primarily due to lower operational costs and favorable exchange rates.
A major advantage of conducting research in South America is the access to skilled professionals at competitive rates. By 2025, the costs associated with a proficient workforce for medical studies are expected to remain substantially lower in Brazil, further establishing the country as a medtech research hub and presenting it as a financially viable option for Medtech firms compared to North America and Europe. This cost-effectiveness is enhanced by the high level of expertise among Brazilian professionals, who are well-versed in the regulatory requirements and practices essential for successful execution.
As Patricio Ledesma, Head of Clinical Operations and Founder at Sofpromed CRO, notes, "The knowledge of local specialists is essential in managing the intricacies of research studies, guaranteeing adherence and effectiveness."
Additionally, Brazil offers a range of governmental incentives aimed at attracting international investment in medical studies. These incentives may include:
- Tax breaks
- Streamlined regulatory processes
These not only lower initial costs but also facilitate faster study initiation and execution. For instance, specific programs may provide financial support for investigative activities or expedited approval processes for medical studies, further enhancing the country's appeal as a destination for research.
Establishing a presence in Brazil as a medtech clinical research hub can yield substantial long-term financial advantages. Companies can leverage local collaborations to boost operational efficiency and mitigate logistical challenges, ultimately leading to improved timelines and outcomes.
Moreover, bioaccess® specializes in comprehensive study management services, encompassing:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project management
- Reporting
With over 20 years of experience in Medtech, bioaccess® possesses the expertise and tailored approach necessary to guide your company towards successful acquisition. Their proficiency in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies positions them as a valuable ally for Medtech firms navigating the complexities of research in Brazil.
A case study on technological advancements in medical studies illustrates how the integration of electronic data capture systems and decentralized research methodologies can further optimize costs. This case study indicates that while there may be an initial investment in these technologies, the long-term savings and enhanced data quality significantly outweigh the upfront expenses. The findings suggest that embracing such innovations can lead to a more efficient testing process, ultimately benefiting both sponsors and participants.
In conclusion, Brazil stands out as a strategic center for medtech clinical research, offering a unique combination of cost advantages, availability of skilled workforce, and supportive government policies that can drive successful studies.
Understanding ANVISA's Role: Opportunities and Challenges
ANVISA's regulatory structure plays a crucial role in shaping the research approval landscape in Brazil. Recent policy changes in 2025 have streamlined processes, facilitating navigation for Medtech companies within the regulatory environment. These modifications aim to enhance efficiency and reduce approval times, positioning Brazil as a premier hub for medtech clinical research and expediting the market entry of innovative medical devices.
Notably, the processing of DDCM requests now takes up to two days for payment clearance, underscoring the advancements in the regulatory process.
The recent updates in ANVISA's policies offer substantial advantages for Medtech companies. These include:
- Simplified submission processes
- Clearer guidelines
These enhancements not only expedite approvals but also alleviate administrative burdens. Such enhancements are vital for companies looking to conduct clinical studies in Brazil, as they facilitate quicker access to the local market and improve patient outcomes.
Nevertheless, regulatory compliance continues to pose challenges for many organizations. To navigate these complexities, companies should invest in comprehensive management services that encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Test setup
- Import permits
- Project oversight
- Reporting
As bioaccess® states, "bioaccess® provides comprehensive management services, including feasibility assessments, site selection, compliance evaluations, trial setup, import permits, project oversight, and reporting, helping researchers navigate the complexities of conducting studies in Brazil."
By partnering with experienced contract development organizations like bioaccess®, Medtech companies can adeptly maneuver through the intricacies of the regulatory landscape, further solidifying Brazil's status as a hub for medtech clinical research.
Collaboration with ANVISA represents another strategic opportunity for Medtech companies to streamline the approval process. Engaging with regulatory bodies early in the development phase can yield valuable insights and foster a more efficient dialogue, ultimately resulting in smoother approvals and enhanced project outcomes.
Staying informed about ANVISA's upcoming initiatives is essential for any Medtech firm involved in trials. As the regulatory landscape evolves, understanding forthcoming changes will empower organizations to adapt their strategies accordingly, ensuring compliance and maximizing the likelihood of successful research studies. Moreover, for protocols involving individuals with extremely rare diseases, sponsors must guarantee access to effective treatments for five years post-study, thereby elevating the ethical standards of medical research and ensuring that participants continue to benefit from effective medical interventions after the study.
Furthermore, bioaccess® is dedicated to data protection and has instituted grievance procedures to address any client concerns, ensuring compliance and transparency throughout the clinical trial process.
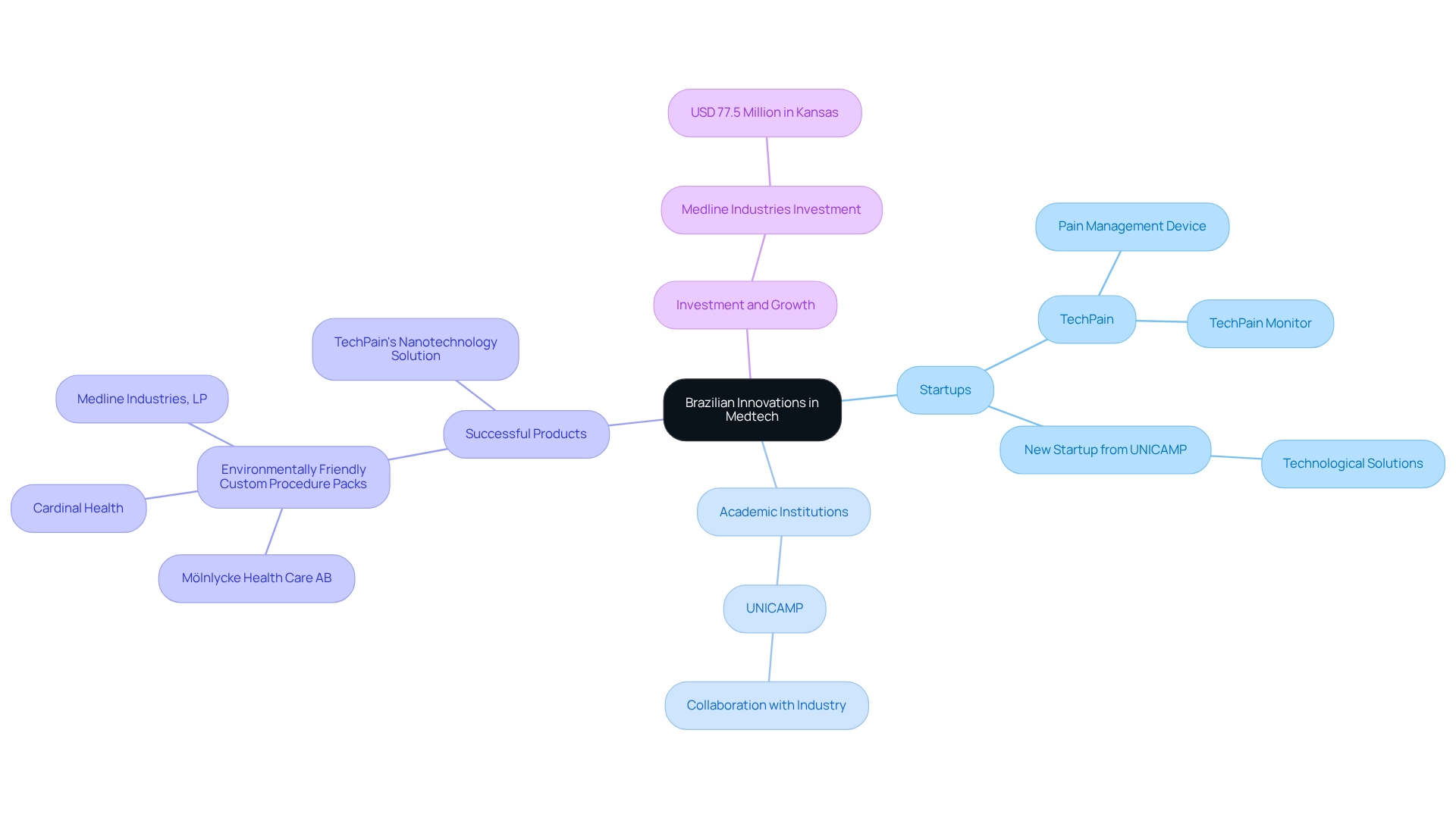
Success Stories: Brazilian Innovations in Medtech
Brazil has emerged as a hub for medtech clinical research, showcasing several companies that successfully launch groundbreaking products to address critical healthcare needs. Notable examples include startups that have developed advanced solutions, such as TechPain's unique pain management device that integrates nanotechnology and software. This innovative technology features a wearable, non-invasive device delivering anti-inflammatory nanomedicine directly to pain sites, controlled by patients via the TechPain Monitor smartphone app.
This highlights Brazil as a hub for medtech clinical research, demonstrating the capability of Brazilian creativity in the Medtech field. Local universities and academic institutions play a vital role in nurturing this innovation ecosystem. Institutions like UNICAMP are not only breeding grounds for new ideas but also work with industry players to convert studies into viable products. This collaboration between academia and industry is essential for promoting progress in medical technology.
Successful trials carried out in Brazil as a hub for medtech clinical research, supported by organizations like bioaccess®, have facilitated product approvals, showcasing the nation's ability to conduct thorough investigations. For instance, the partnership between Medtech companies and local universities has led to the development of environmentally friendly healthcare solutions, such as sustainable custom procedure packs. Mölnlycke Health Care AB's initiative to create eco-friendly products underscores a growing trend towards sustainability in the healthcare sector, addressing both medical needs and environmental concerns.
Several factors contribute to the success of these initiatives, including strategic collaborations, significant investments, and a supportive regulatory environment. The recent investment of USD 77.5 million by Medline Industries in a distribution center in Kansas reflects the growing confidence in the Latin American market, which is increasingly acknowledged for its potential in clinical studies.
To enhance study outcomes, it is essential for Medtech companies to forge partnerships with local innovators and academic institutions. By leveraging the expertise and resources available in Brazil as a hub for medtech clinical research, companies can accelerate their product development processes and achieve successful market entry. This collaborative approach not only enriches the research landscape but also ensures that innovations are tailored to meet the specific needs of the local healthcare system.
With more than 20 years of expertise in Medtech, bioaccess® pioneers comprehensive trial management services, focusing on Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). The future of Medtech in the country appears promising.
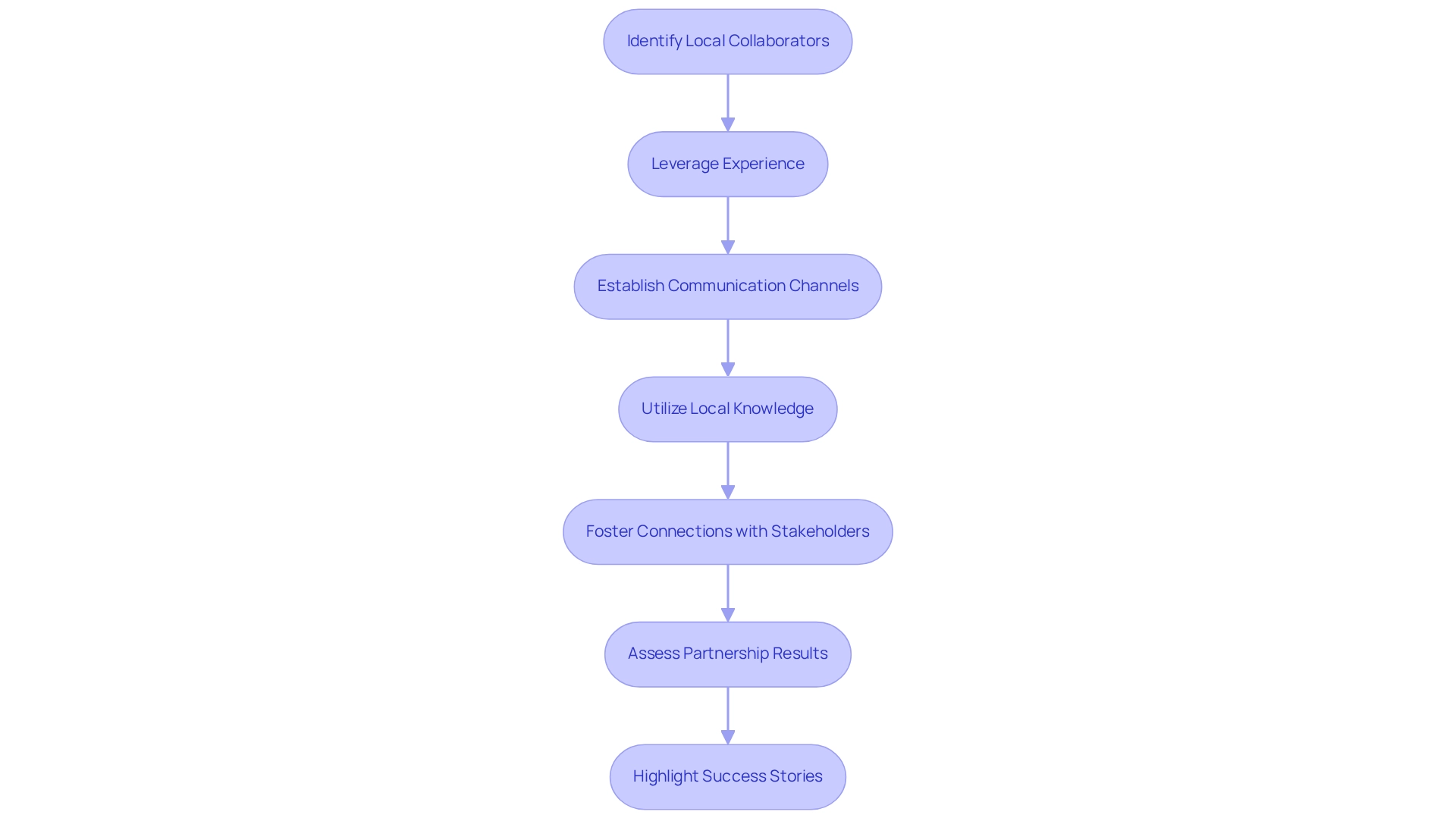
Building Strategic Partnerships for Success in Brazil
Identify potential local collaborators, including Contract Research Organizations (CROs), academic institutions, and healthcare providers, to enhance the effectiveness of trials. Leveraging bioaccess®'s 20+ years of experience in managing various types of studies—such as Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF)—can significantly improve partnership outcomes. Establishing clear communication channels and shared objectives with partners fosters collaboration and ensures all parties are aligned in their goals. Effective collaborations, like those with Welwaze Medical Inc. for the Celbrea® launch in Colombia, showcase the efficacy of strategic alliances in securing regulatory access and market entry.
Utilize local knowledge to navigate the regulatory environment and cultural subtleties, which can greatly influence the success of trials. The Latin American Cooperative Oncology Group demonstrates effective partnership among medical oncologists throughout Latin America, tackling distinct health challenges via a collaborative method. Foster connections with key stakeholders, including government agencies and industry associations, to obtain backing and resources that can enhance investigative initiatives. As highlighted by Clarissa Mathias, MD, PhD, in her observations on establishing a medical investigation network in South America, strategic collaborations are essential for addressing obstacles.
Consistently assess partnership results to guarantee they align with study goals, enabling modifications and enhancements in strategy as required. Efficient operational processes, such as the timely processing of DDCM requests, are essential for maintaining effective partnerships. Highlighting success stories from previous collaborations in Brazil as a hub for medtech clinical research, such as those facilitated by bioaccess®, can illustrate the potential benefits of strategic partnerships. The partnership with GlobalCare Clinical Trials, which accomplished over a 50% decrease in recruitment time and 95% retention rates, serves as a compelling example of how effective collaborations can drive success in medical studies.
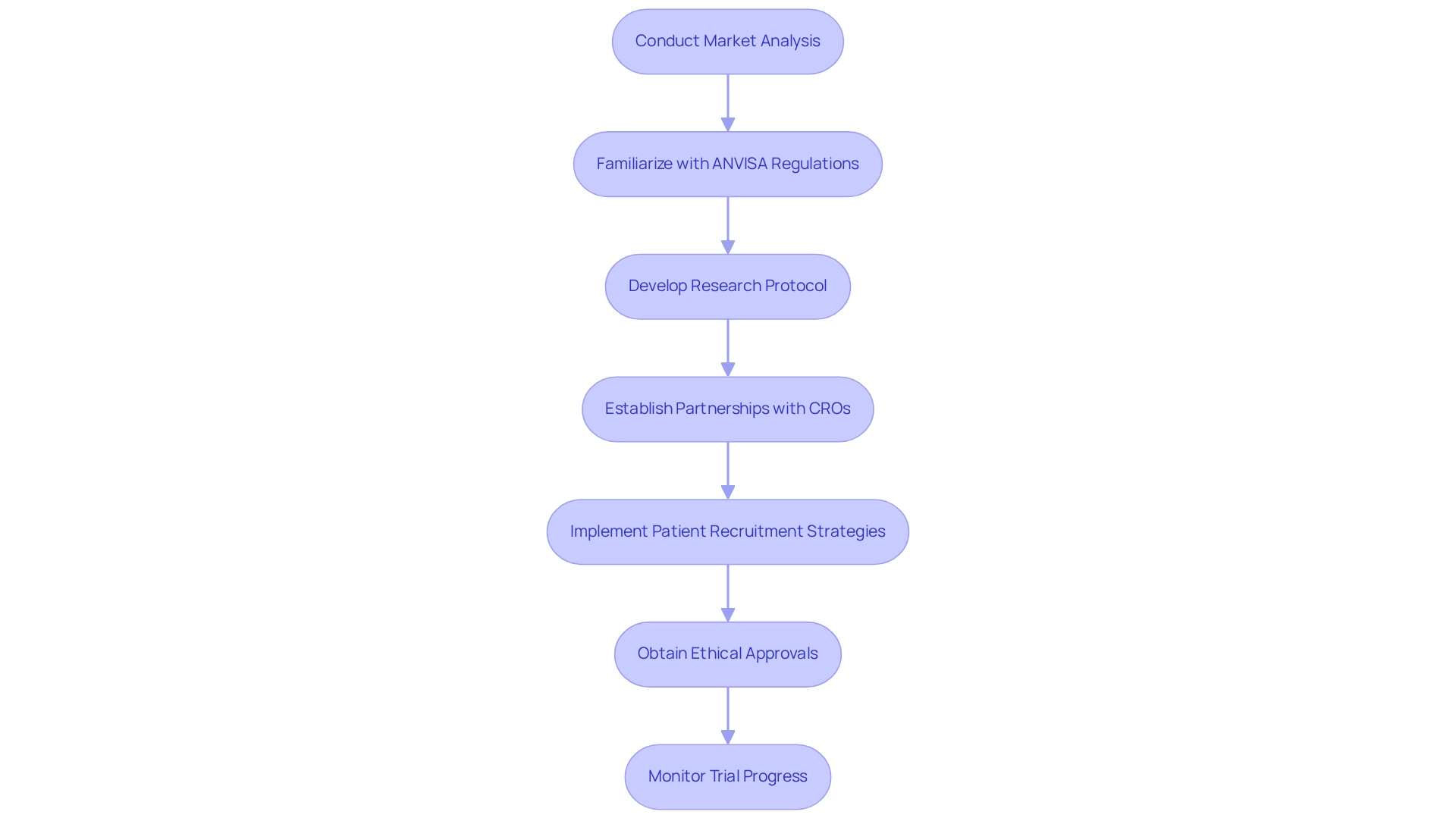
Your Essential Checklist for Conducting Clinical Research in Brazil
Conduct a comprehensive market analysis to gain insights into Brazil as a hub for medtech clinical research. This analysis should identify key trends, opportunities, and challenges that may impact your research initiatives. Notably, more than half of vendors (53%) that accept payments at the point of sale are planning to implement or are considering connected car payment options, indicating a broader technological shift that may influence Medtech innovations.
Familiarize yourself with ANVISA's regulations and compliance requirements, which are essential for the successful execution of research in Brazil. Understanding these guidelines will simplify the approval procedure and ensure compliance with local standards.
Develop a robust research protocol that supports Brazil as a hub for medtech clinical research while aligning with both local regulations and international best practices. This protocol should clearly outline the study's objectives, methodology, and compliance measures to facilitate a smooth review process.
Establish strategic partnerships with local contract organizations (CROs) like bioaccess®, which specializes in facilitating medical device evaluations in Latin America. This collaboration will further establish Brazil as a hub for medtech clinical research, encompassing:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies
Working with experienced local entities can enhance operational efficiency and provide valuable insights into the regional healthcare ecosystem. It is crucial to implement effective patient recruitment strategies tailored to the Brazilian population, considering cultural nuances and local healthcare access to optimize recruitment efforts and ensure diverse participant representation. As highlighted by Michael Wolf, average earnings were up 9.6% in August 2024, which may positively impact participant recruitment and funding availability.
Ensure that ethical approvals are obtained from relevant committees, including Institutional Review Boards (IRBs). This step is vital for maintaining the integrity of your research and safeguarding participant rights. Monitor trial progress closely and be prepared to adapt strategies as needed to address any challenges that arise. Flexibility in your approach can significantly enhance the likelihood of successful outcomes in Brazil as a hub for medtech clinical research. Additionally, consider the case study on investment in low-carbon technologies, which illustrates how companies are adapting to market changes—an approach that can parallel the Medtech sector's need for innovation and adaptation in clinical research.
As you navigate these processes, remember that bioaccess® is committed to ensuring information security and client trust. They have established grievance and data protection procedures to address any concerns related to compliance and transparency.
Conclusion
Brazil is emerging as a prominent hub for Medtech clinical research, bolstered by its strategic location, diverse patient demographics, and ongoing enhancements in regulatory frameworks. This article underscores Brazil's dedication to improving its healthcare infrastructure and regulatory processes, particularly through organizations like ANVISA, which are attracting international sponsors and facilitating successful clinical trials. The combination of cost efficiency, a treatment-naïve population, and ethical considerations further solidifies Brazil's status as an ideal destination for clinical research.
The benefits of conducting clinical trials in Brazil are evident: substantial cost savings, access to a skilled workforce, and a rich pool of diverse participants contribute to more robust data collection and outcomes. Furthermore, the emphasis on strategic partnerships with local CROs and research institutions, such as bioaccess®, is critical in navigating the complexities of the Brazilian regulatory landscape. These collaborations not only enhance operational efficiency but also ensure that innovations are tailored to meet the unique needs of the local healthcare system.
As the Medtech sector in Brazil continues to progress, the opportunities for innovation and successful clinical trials are expanding. With a supportive regulatory environment, a commitment to ethical practices, and a focus on collaboration, Brazil is well-positioned to propel advancements in medical technology. By embracing these factors, Medtech companies can capitalize on Brazil's potential, ultimately improving patient outcomes both locally and globally.
Frequently Asked Questions
Why is Brazil considered a hub for medtech clinical research?
Brazil is strategically located in Latin America, making it a pivotal hub for medtech clinical research. Its commitment to enhancing healthcare and regulatory systems, along with recent investments in healthcare infrastructure, significantly boosts its appeal for conducting medical studies.
What are the costs associated with conducting medical research in Brazil?
Sponsors are required to pay a Health Surveillance Inspection Fee when submitting a research application, with fees ranging from 983.85 to 19,677 Brazilian Reals.
How is Brazil's patient demographic beneficial for medical studies?
Brazil's diverse patient demographic provides a rich database that enhances the strength and relevance of findings across various populations, allowing for a more comprehensive understanding of medical conditions and treatments.
What ethical considerations are important in Brazilian clinical trials?
Ethical considerations are paramount, including the requirement for approval from an ethics committee when involving participants who cannot provide consent. This ensures participants' rights are respected and they are adequately informed about the study.
What role does ANVISA play in Brazil's medtech research landscape?
ANVISA's membership in PIC/S and adherence to globally harmonized GMP inspection standards highlight Brazil’s commitment to maintaining high regulatory standards in medical studies, which fosters successful international collaborations.
What recent law has impacted the approval process for medical studies in Brazil?
Law No. 14.874, enacted in May 2024, significantly simplifies the approval process for medical studies, aiming to reduce bureaucratic obstacles and accelerate the introduction of innovative medical technologies to the marketplace.
How can researchers navigate compliance challenges in Brazil?
Engaging with local regulatory specialists can provide essential insights into navigating complex compliance challenges, helping to mitigate risks and enhance the efficiency of the approval process.
What is the potential impact of the FDA's user fees on research in Brazil?
Understanding the implications of the FDA's user fees for New Drug Applications can offer a broader perspective on the regulatory framework that may influence Brazil as a hub for medtech clinical research.
What services does bioaccess® provide to support medtech startups?
Bioaccess® offers affordable services, including regulatory approval, research site activation, subject recruitment, and data management, helping medtech startups transition smoothly to the next phase of studies and bolster fundraising efforts.




