Overview
Selecting research sites in Latin America is crucial for the success of clinical studies, as it profoundly influences patient recruitment, compliance with local regulations, and overall study outcomes. This article highlights that strategic site selection—guided by factors such as local healthcare infrastructure, historical performance, and community partnerships—can boost trial success rates by as much as 30%. This underscores the pivotal role of site selection in advancing effective medical research in the region.
Introduction
In the evolving landscape of clinical research, site selection emerges as a critical determinant of success, particularly within the diverse and dynamic context of Latin America. This region, characterized by a rich tapestry of populations and regulatory frameworks, presents unique challenges and opportunities. The choice of research sites can significantly influence patient recruitment, retention, and overall study outcomes.
As the region prepares for a surge in medical studies, understanding the nuanced factors that contribute to effective site selection becomes imperative. Evaluating local healthcare systems and fostering community partnerships are strategies that extend beyond logistics; they enhance the integrity and impact of clinical trials.
As organizations like bioaccess® lead the charge in navigating these complexities, the potential for groundbreaking advancements in global health becomes increasingly attainable.
The Significance of Site Selection in Latin American Research
Choosing research sites in Latin America is crucial in the study process, particularly due to the environment's diverse populations and varying regulatory frameworks. A strategically selected location not only streamlines patient recruitment but also ensures adherence to local regulations, significantly influencing the overall success of the study.
In 2025, the selection of research sites in Latin America for clinical studies is of paramount importance. Factors such as the facility's infrastructure, access to intended patient groups, and previous performance in earlier studies are essential for making informed choices. Locations with robust infrastructure and established connections within the regional healthcare system can markedly improve patient recruitment and retention, ultimately leading to more favorable study outcomes.
Statistics indicate that effective site selection can enhance trial success rates by up to 30%, underscoring the necessity for meticulous planning and evaluation. Furthermore, expert insights emphasize that comprehending regional healthcare systems and fostering relationships with nearby stakeholders are critical for improving market access and ensuring the success of research studies. As Steve Garchow aptly stated, "Comprehending regional healthcare systems and fostering connections with community stakeholders to enhance market access and success in studies" is vital for achieving desired outcomes.
The partnership between bioaccess™ and Caribbean Health Group exemplifies this approach, aiming to establish Barranquilla as a premier location for trials in Latin America. Supported by Colombia's Minister of Health, this initiative not only enhances regional capabilities but also drives international collaboration, ultimately benefiting global health outcomes.
Case studies illustrate the dynamic landscape of medical research in Latin America. One notable example discusses the challenges and opportunities faced in the region, including regulatory hurdles, infrastructure limitations, and cultural differences that can impede study success. By addressing these challenges and leveraging local knowledge, stakeholders can enhance collaboration and effectiveness in research, contributing to advancements in global health.
For instance, GlobalCare Clinical Trials, in partnership with bioaccess™, achieved over a 50% reduction in recruitment time and a 95% retention rate, showcasing the effectiveness of their collaborative approach.
In summary, the influence of location choice on patient recruitment and study outcomes is profound. As the Medtech industry continues to advance, selecting research sites in Latin America will be crucial for attaining positive research outcomes. With over 15 years of experience in the Medtech sector, bioaccess® provides extensive research management services, including feasibility studies, location selection, compliance reviews, project setup, import permits, project management, and reporting, ensuring that organizations navigate the intricacies of location selection effectively.
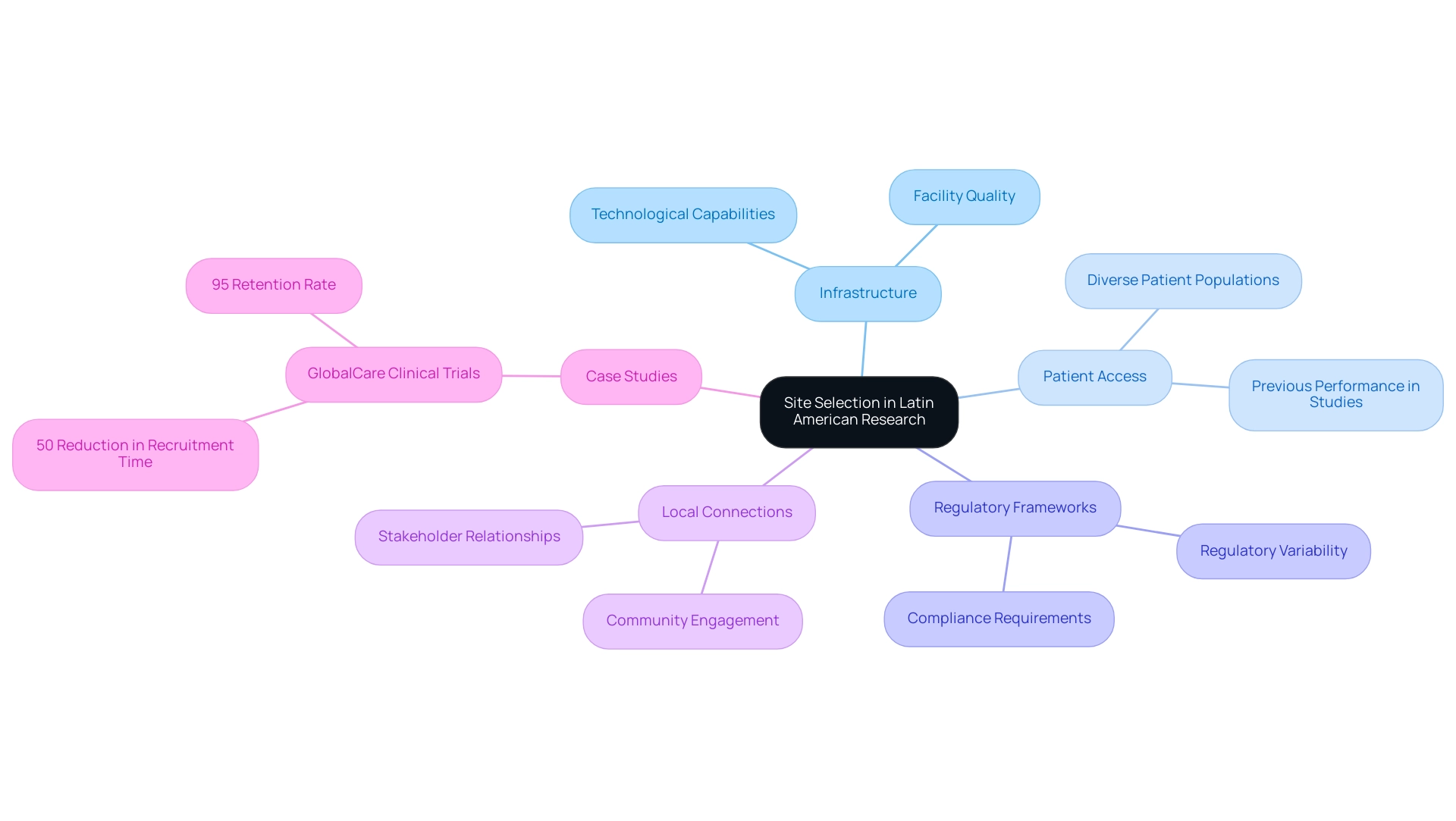
Key Considerations for Selecting Research Sites
When selecting research locations in Latin America, several critical factors must be meticulously assessed to ensure the success of clinical studies.
- Patient Population: Understanding the demographics and prevalent health conditions in the region is crucial. This alignment with the study's target population enhances recruitment efforts and ensures that the findings are relevant and applicable to the intended patient group. With a diverse population across Latin America, tailoring studies to reflect local health challenges can significantly improve patient outcomes. As Mirella Nardo from ICESP points out, despite advancements in cancer prevention, challenges in obtaining sufficient treatment persist in economically disadvantaged nations, underscoring the necessity of considering patient demographics during location selection.
- Location Experience: The historical performance of a location in executing comparable studies is another vital factor. Evaluating past recruitment success rates and adherence to study protocols offers insights into the location's reliability and capability. Sites with a proven track record are more likely to navigate challenges effectively, ensuring smoother execution. With over 15 years of experience in the Medtech industry, bioaccess® understands the subtleties of site selection and the significance of leveraging historical data.
- Infrastructure: Adequate facilities and equipment are essential for the efficient execution of research studies. Sites must be equipped to handle the specific requirements of the study, including laboratory capabilities, patient care facilities, and data management systems. This infrastructure supports not only the operational aspects of the study but also the safety and comfort of participants.
- Regulatory Knowledge: A comprehensive understanding of local regulatory requirements is imperative for compliance throughout the study. Sites that are well-versed in the regulatory landscape can facilitate smoother interactions with authorities, ensuring that all necessary approvals are obtained promptly. This knowledge is particularly important in a region where regulations are evolving to keep pace with advancements in medical technology. For instance, Colombia’s INVIMA serves as a Level 4 health authority by PAHO/WHO, overseeing medical device regulation, reflecting the dynamic regulatory environment. Colombia's science, technology, and innovation strategy for 2022–2031 aims to strengthen its role in global research, making regulatory knowledge even more pertinent.
As Latin America positions itself as a competitive player in global health studies by 2025, these factors will be pivotal in the successful execution of clinical trials. The anticipated growth in medical studies, driven by increased investment and technological integration, underscores the significance of choosing research sites in Latin America to foster innovation and improve patient outcomes. Emerging fields such as gene therapy and digital health interventions present new opportunities for innovation in the region, further reinforcing the need for well-equipped research sites. By effectively managing these challenges, bioaccess® establishes itself as a prominent contract research organization, supporting medical device studies through extensive services including setup, project management, and reporting. This not only contributes to regional economies through job creation and improved healthcare access but also aligns with the expected growth in medical studies across the area.
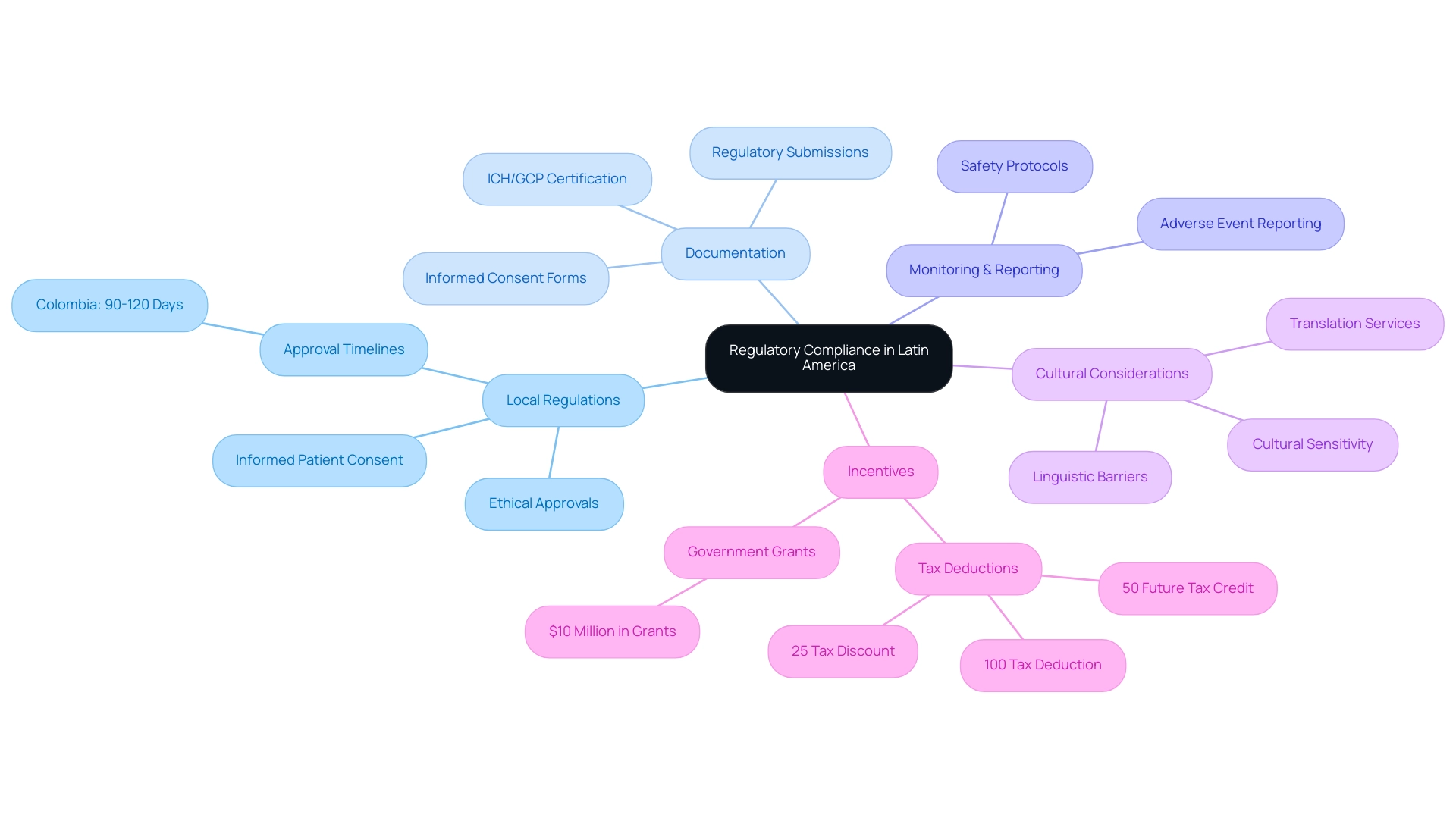
Understanding Regulatory Compliance in Latin America
Choosing research sites in Latin America is a critical endeavor that involves navigating complex regulatory compliance, as regulations vary significantly across countries. Researchers must be well-versed in several essential areas.
Local regulations are paramount; each nation has distinct laws governing clinical trials, which include requirements for ethical approvals and informed patient consent. In Colombia, for instance, the IRB/EC and INVIMA approval processes can be completed in just 90-120 days, significantly faster than in many other regions. Understanding these ethical approval processes is vital when selecting research sites in Latin America, as they can directly impact the timeline and feasibility of studies.
Documentation is another key factor. It is essential to prepare and submit all necessary documentation in strict accordance with regional guidelines. This encompasses regulatory submissions, informed consent forms, and any additional materials required by local authorities. Colombia’s healthcare system, ranked within the top five globally, mandates that hospitals conducting clinical research adhere to rigorous ICH/GCP certification processes. Comprehensive documentation is critical for compliance; failing to follow these requirements can lead to delays or even rejection of application submissions.
Monitoring and reporting also play a crucial role. Researchers must grasp the specific requirements for monitoring progress and reporting adverse events to regulatory bodies. This includes adhering to regional protocols for safety reporting, which are vital for maintaining participant safety and ensuring compliance with ethical standards.
Moreover, addressing linguistic and cultural barriers is significant when choosing research sites in Latin America. Effective translation of regulatory and patient-related materials is necessary to avoid misunderstandings and delays in the informed consent process. Properly addressing these differences is crucial for the ethical treatment and safety of participants, underscoring the importance of culturally sensitive translation services.
Colombia offers substantial incentives for investments in science and technology, including a 100% tax deduction, a 25% tax discount, a 50% future tax credit, and approximately $10 million in government grants. Such initiatives not only encourage clinical research but also enhance the overall quality of trials conducted in the region. As Dr. John B. Simpson observed, "Collaborating with LATAM CRO experts who comprehend regional market dynamics is essential."
Choosing research sites in Latin America, particularly in Colombia with its population exceeding 50 million and 95% coverage under universal healthcare, is crucial for patient recruitment. Researchers must stay informed about local regulations and compliance requirements. The large population base and universal healthcare coverage facilitate participant enrollment and ensure a robust recruitment process. Bioaccess provides comprehensive trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—positioning itself as a valuable partner in navigating these complexities.
Logistical Considerations for Research Execution
Choosing research sites in Latin America involves crucial logistical considerations for the successful execution of medical research. Key aspects to focus on include:
- Recruitment Strategies: Implement targeted recruitment strategies that tap into local networks and foster community engagement. This method not only draws in participants but also improves the overall quality of medical studies. In 2025, effective recruitment strategies are more crucial than ever, as studies show that experiments with strong community involvement experience a 30% rise in participant retention rates. Additionally, addressing linguistic, cultural, and socio-economic barriers is essential to improve the quality of clinical trials and protect patients.
- Community Partnerships: Establishing alliances with regional healthcare providers and organizations is crucial for enhancing recruitment efforts. These collaborations help build community trust, which is critical in navigating the socio-economic and cultural landscape of Latin America. Involving regional experts can significantly enhance the recruitment process, as they possess insights into community dynamics and patient preferences. As Dr. John B. Simpson states, "Collaborating with LATAM CRO experts who understand local market dynamics is essential."
- Supply Chain Management: Efficient supply chain management is necessary to ensure that all required supplies and equipment are available at the research site. Delays in execution can arise from logistical oversights, which can be mitigated by establishing reliable supply chains. In 2025, the complexity of regulatory requirements necessitates that sponsors maintain a proactive approach to logistics, ensuring that all materials are on hand to facilitate smooth operations.
- Cultural Competence: Comprehending regional customs and practices is essential for successful implementation in medical studies. A case study titled "Cultural Competence in Clinical Research" highlights that CROs exhibiting cultural competence, like bioaccess®, enhance participant engagement and study performance. By addressing these logistical considerations, research organizations can enhance participant involvement and optimize study performance, ultimately leading to more successful results, especially when choosing research sites in Latin America. Moreover, bioaccess® provides extensive study management services across different phases, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), ensuring effective navigation of the local regulatory environment as overseen by INVIMA, Colombia's Level 4 health authority. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, attests to bioaccess®'s expertise, sharing his positive experience during its initial human study in Colombia.
Evaluating Potential Research Sites: Criteria and Best Practices
When evaluating potential research sites in Latin America, it is essential to consider several key criteria that can significantly impact the success of clinical trials:
- Historical Performance: Analyzing a site's historical performance is crucial. This includes reviewing metrics such as patient recruitment rates and adherence to timelines. Locations with a demonstrated history, as shown by bioaccess®, which has over 20 years of experience in overseeing research studies, can offer valuable insights into their operational efficiency and reliability.
- Staff Qualifications: The qualifications and experience of the research staff, particularly principal investigators and coordinators, are vital. Evaluating their backgrounds, training, and past involvement can help ensure that the team is prepared to manage the complexities of medical research.
- Facility Readiness: A thorough inspection of the facilities is necessary to confirm they meet the required standards for conducting clinical trials. This includes evaluating the availability of necessary equipment, technology, and compliance with regulatory requirements.
- Patient Access: The platform's ability to effectively reach the target patient population is another critical factor. Understanding the demographics and health conditions prevalent in the area can help assess the location's potential for successful patient recruitment.
In 2025, as sponsors increasingly seek to consolidate data management and gain ownership of their data, these criteria become even more pertinent. Leianne Ebert, Head of Clinical Data Operations at Alcon, noted, "This is something we monitor regularly, and last week, our records showed that 45% of our data is entered on the same day as the visit date." This emphasizes the significance of prompt data management in medical studies.
The shift towards insourced models and the need for technology that permits direct access to live data highlight the significance of choosing research sites in Latin America that not only satisfy operational standards but also correspond with the changing environment of research. Moreover, bioaccess's partnership with Caribbean Health Group to establish Barranquilla as a top destination for medical studies, backed by Colombia's Minister of Health, emphasizes the importance of choosing research sites in Latin America, reflecting the region's growing potential in medical research. By adhering to these best practices and leveraging bioaccess's expertise in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, organizations can enhance their site evaluation processes, ultimately leading to more successful clinical trials in the region.
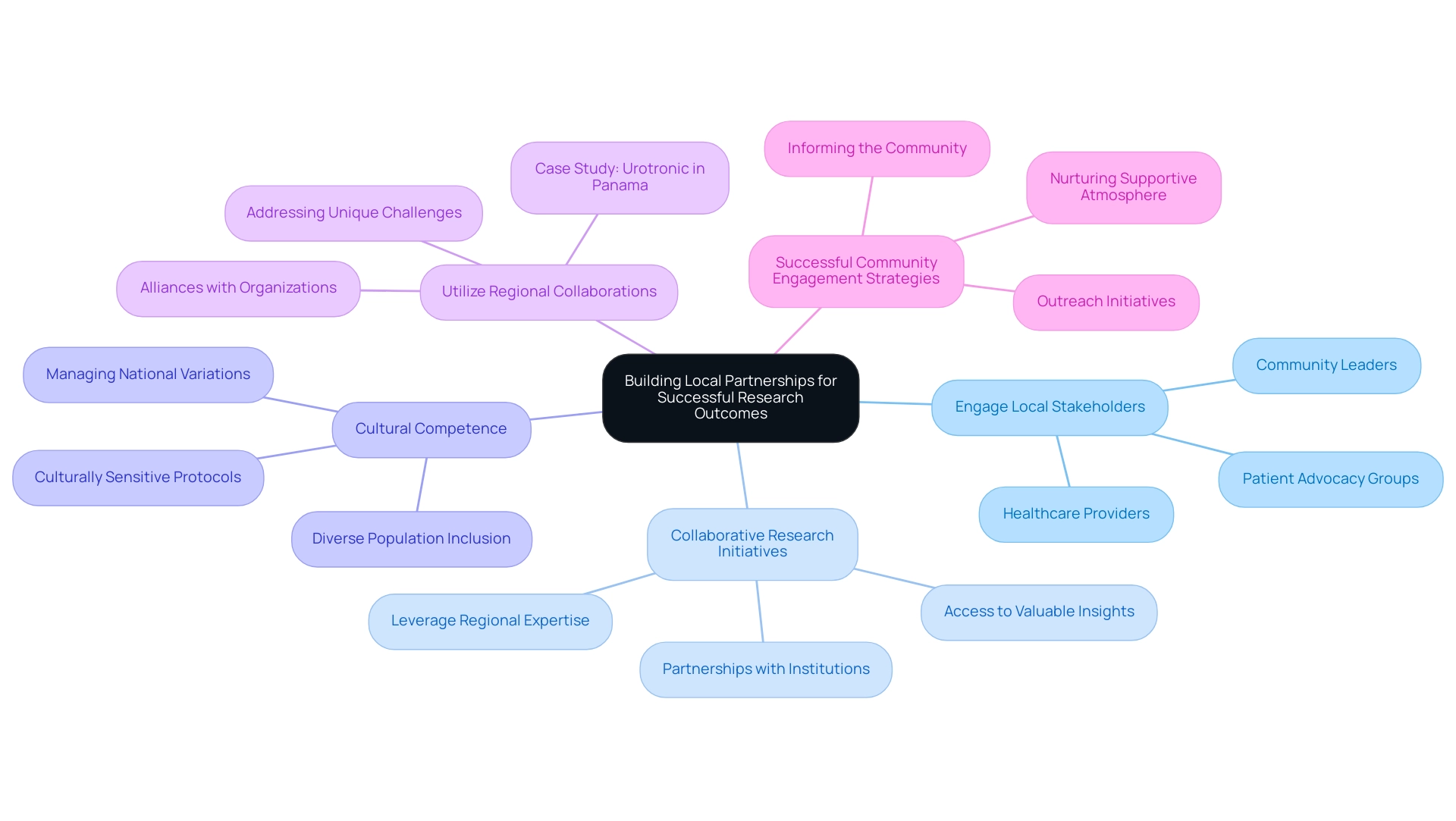
Building Local Partnerships for Successful Research Outcomes
Choosing research sites in Latin America is essential for establishing regional partnerships that lead to successful research outcomes. To achieve this, several effective strategies should be considered:
- Engage Local Stakeholders: Actively identify and engage with local stakeholders, including healthcare providers, community leaders, and patient advocacy groups. Establishing strong relationships with these entities facilitates smoother trial processes and enhances community trust. As Renata Berardocco, Executive Vice President and Managing Director for LATAM, states, "I believe patient recruitment is the key to realizing these benefits, and I know from experience that Brazil, Mexico, and Argentina, in particular, have large patient populations and access to exceptional Principal Investigators."
- Collaborative Research Initiatives: Seek opportunities for collaborative research initiatives that leverage regional expertise and resources. By partnering with regional institutions, researchers can access valuable insights and networks that enhance study design and execution. bioaccess® specializes in comprehensive research management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF), ensuring thorough oversight throughout the process.
- Cultural Competence: Ensuring that research protocols are culturally sensitive and customized to the regional context is essential. This approach not only enhances participant recruitment and retention but also fosters a more inclusive environment for diverse populations. Preparing for variations in national infrastructures, languages, and cultures can help manage expenses and cycle times in research studies.
- Utilize Regional Collaborations: Establishing alliances with regional organizations significantly influences research outcomes. For instance, bioaccess® successfully managed Urotronic's first-in-human study of the Optilume drug-coated balloon in Panama, demonstrating the region's potential as a viable site for Medtech research, despite its smaller size and limited recruitment capabilities. Such case studies emphasize the significance of local partnerships in choosing research sites in Latin America to address unique challenges.
- Successful Community Engagement Strategies: Implementing effective community engagement strategies is vital. This encompasses organizing outreach initiatives that inform the community about the research process and its advantages, thereby nurturing a supportive atmosphere for medical studies.
With over 20 years of experience in Medtech, bioaccess® understands the significance of these strategies. Under the leadership of Julio Martinez-Clark, CEO, who advocates for Medtech research in Latin America, and Monica Mora, COO, who specializes in operations and regulatory strategies, researchers can enhance their trial outcomes and contribute to the growth of the Medtech sector in the region, ultimately improving healthcare delivery. Additionally, Medtech studies positively influence local economies through job creation, economic growth, and enhanced healthcare services, fostering international collaboration.
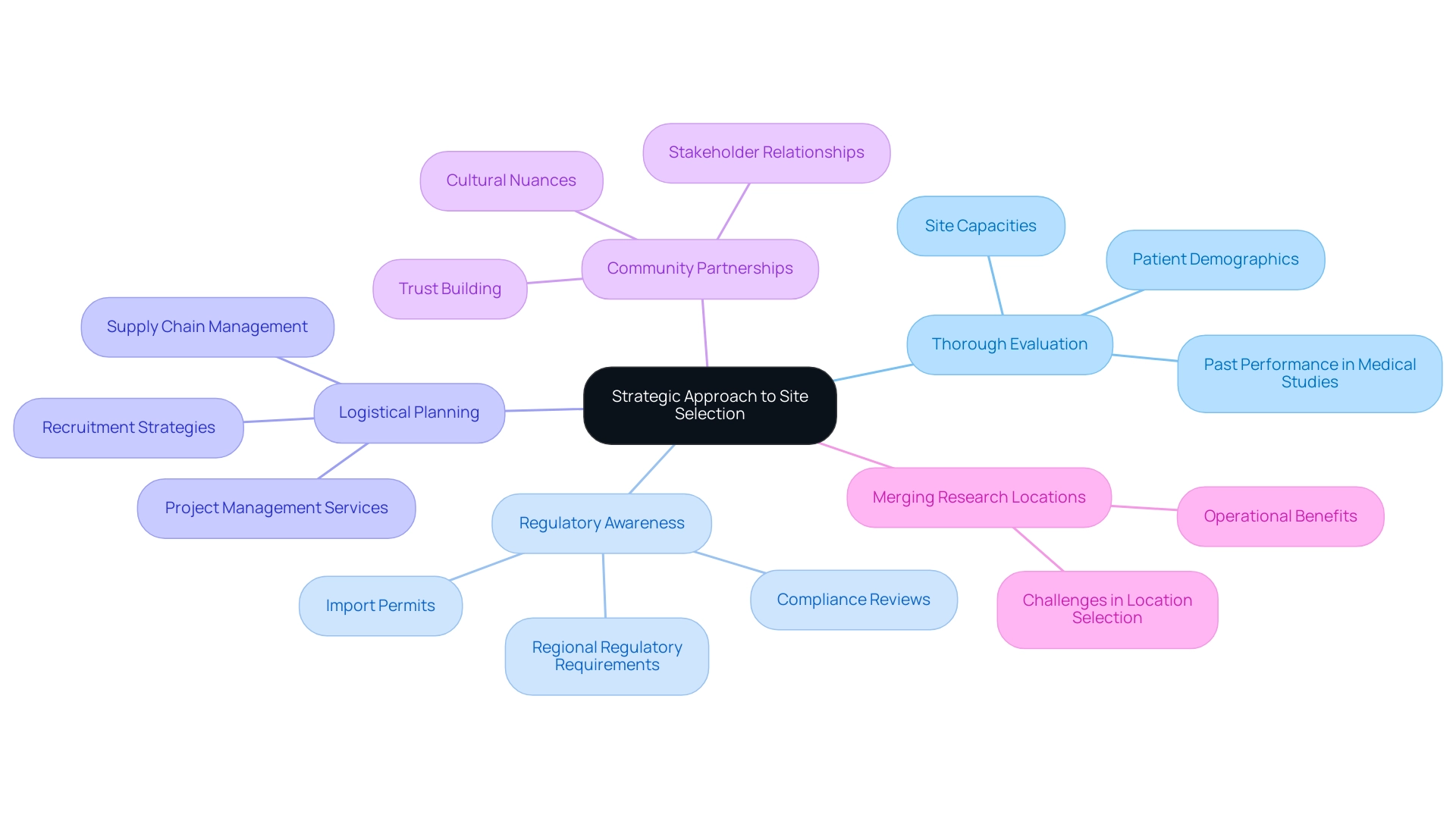
Strategic Approach to Site Selection: A Summary
An effective site selection process in Latin America encompasses several strategic elements:
- Thorough Evaluation: A comprehensive assessment of potential sites is essential. This entails examining key factors such as site capacities, patient demographics, and past performance in medical studies. A meticulous evaluation can significantly enhance the chances of success, especially when supported by bioaccess's expertise in delivering high-quality clinical research services.
- Regulatory Awareness: Keeping informed about regional regulatory requirements is essential for ensuring compliance and facilitating smoother operational processes. Understanding the evolving regulatory landscape in countries like Peru, Colombia, and Chile is vital. Bioaccess's knowledge in navigating these complexities, including compliance reviews and import permits, helps mitigate risks associated with non-compliance.
- Logistical Planning: Robust logistical strategies are vital to address recruitment challenges and supply chain complexities. Effective planning ensures that trials can proceed without unnecessary delays, ultimately improving patient recruitment and retention rates. Utilizing bioaccess's project management services can streamline these processes.
- Community Partnerships: Establishing strong relationships with regional stakeholders, including healthcare providers and regulatory bodies, can enhance research outcomes. As Ashley Davidson, Vice President of Product Lead - Sponsor Tech Strategy, noted, "There is a need for more site-centric approaches in study startup." Collaborating with local experts not only aids in navigating cultural nuances but also fosters trust within the community, which is essential for patient engagement.
The merging of research locations signifies a notable change in the industry, providing operational benefits while also introducing obstacles that need to be tackled during location selection. Furthermore, as the number of outsourced research studies rises in Peru, Colombia, and Chile, sponsors must address linguistic, cultural, and socio-economic obstacles to enhance patient protection and study quality.
By applying these strategic measures, researchers can improve their selection process by choosing research sites in Latin America and utilizing bioaccess's extensive management services for studies, thus boosting the chances of successful studies. The technological and service market for studies in the region is anticipated to attain USD 5,920.7 million by 2030, indicating an increasing demand for effective research processes and enhanced access to medical investigations. This underscores the importance of a well-planned site selection strategy in capitalizing on the opportunities presented by this expanding market.
Additionally, bioaccess's commitment to reporting on study status, inventory, and adverse events further enhances its support for successful clinical trials.
Conclusion
Selecting the right research sites in Latin America is a pivotal aspect of conducting successful clinical trials. The insights gathered throughout this article highlight the significance of thorough evaluations that consider patient demographics, site experience, infrastructure, and regulatory knowledge. These elements not only facilitate patient recruitment but also ensure adherence to local regulations, ultimately enhancing study outcomes.
As the region continues to evolve into a competitive player in global health research, the importance of strategic site selection becomes ever more pronounced. Collaborations with local stakeholders and a deep understanding of the cultural and logistical landscape are essential for navigating the complexities of clinical trials. Organizations like bioaccess® exemplify the value of local partnerships and expertise in fostering community trust and optimizing research execution.
In conclusion, the future of clinical trials in Latin America is bright, driven by the potential for innovation and growth. By prioritizing effective site selection and leveraging local knowledge, researchers can unlock new opportunities for advancements in medical science. As the demand for efficient trial processes increases, the commitment to thorough planning and collaboration will be crucial in achieving successful outcomes and improving global health.
Frequently Asked Questions
Why is choosing research sites in Latin America important for clinical studies?
Selecting research sites in Latin America is crucial due to the region's diverse populations and varying regulatory frameworks, which can streamline patient recruitment and ensure adherence to local regulations, significantly influencing the study's overall success.
What factors should be considered when selecting research sites in Latin America?
Key factors include the facility's infrastructure, access to intended patient groups, previous performance in earlier studies, understanding local demographics, and knowledge of regulatory requirements.
How can effective site selection impact trial success rates?
Effective site selection can enhance trial success rates by up to 30%, highlighting the importance of meticulous planning and evaluation in the selection process.
What role does understanding regional healthcare systems play in research studies?
Comprehending regional healthcare systems and fostering relationships with local stakeholders are critical for improving market access and ensuring the success of research studies.
Can you provide an example of a partnership aimed at enhancing research site selection in Latin America?
The partnership between bioaccess™ and Caribbean Health Group aims to establish Barranquilla as a premier location for trials in Latin America, supported by Colombia's Minister of Health to enhance regional capabilities and drive international collaboration.
What challenges do researchers face in Latin America regarding clinical trials?
Researchers face challenges such as regulatory hurdles, infrastructure limitations, and cultural differences that can impede study success.
How did GlobalCare Clinical Trials demonstrate effective collaboration in site selection?
GlobalCare Clinical Trials, in partnership with bioaccess™, achieved over a 50% reduction in recruitment time and a 95% retention rate, showcasing the effectiveness of their collaborative approach.
What services does bioaccess® provide to assist with research site selection?
Bioaccess® offers extensive research management services, including feasibility studies, location selection, compliance reviews, project setup, import permits, project management, and reporting.
Why is infrastructure important for research sites?
Adequate facilities and equipment are essential for efficiently executing research studies, ensuring safety and comfort for participants, and supporting operational aspects like laboratory capabilities and data management systems.
How does regulatory knowledge affect clinical studies in Latin America?
A comprehensive understanding of local regulatory requirements is imperative for compliance, facilitating smoother interactions with authorities and ensuring timely approvals throughout the study.




