Overview
This article outlines the essential steps and considerations for designing trials for wearable devices in Chile, highlighting the critical importance of understanding the local regulatory framework and ethical standards. It emphasizes that adherence to the regulations established by the Chilean Public Health Institute (ISP) and ethical practices in participant engagement are vital for ensuring compliance and the success of clinical studies. Such factors significantly influence the integrity and effectiveness of wearable technology in healthcare research, underscoring the need for a thorough grasp of these elements in the clinical research landscape.
Introduction
In the rapidly evolving landscape of wearable technology, conducting clinical trials in Chile presents a unique set of challenges and opportunities. Navigating the intricate regulatory framework established by the Chilean Public Health Institute and other authorities is crucial for researchers aiming to ensure ethical compliance and participant safety. As the demand for innovative healthcare solutions grows, understanding the roles of key regulatory bodies and adhering to best practices in trial design becomes paramount. This article delves into the essential components for successfully conducting wearable device trials in Chile, from regulatory considerations to ethical implications, ultimately shedding light on how these efforts can enhance healthcare delivery and foster technological advancements.
Understand the Regulatory Framework for Wearable Device Trials in Chile
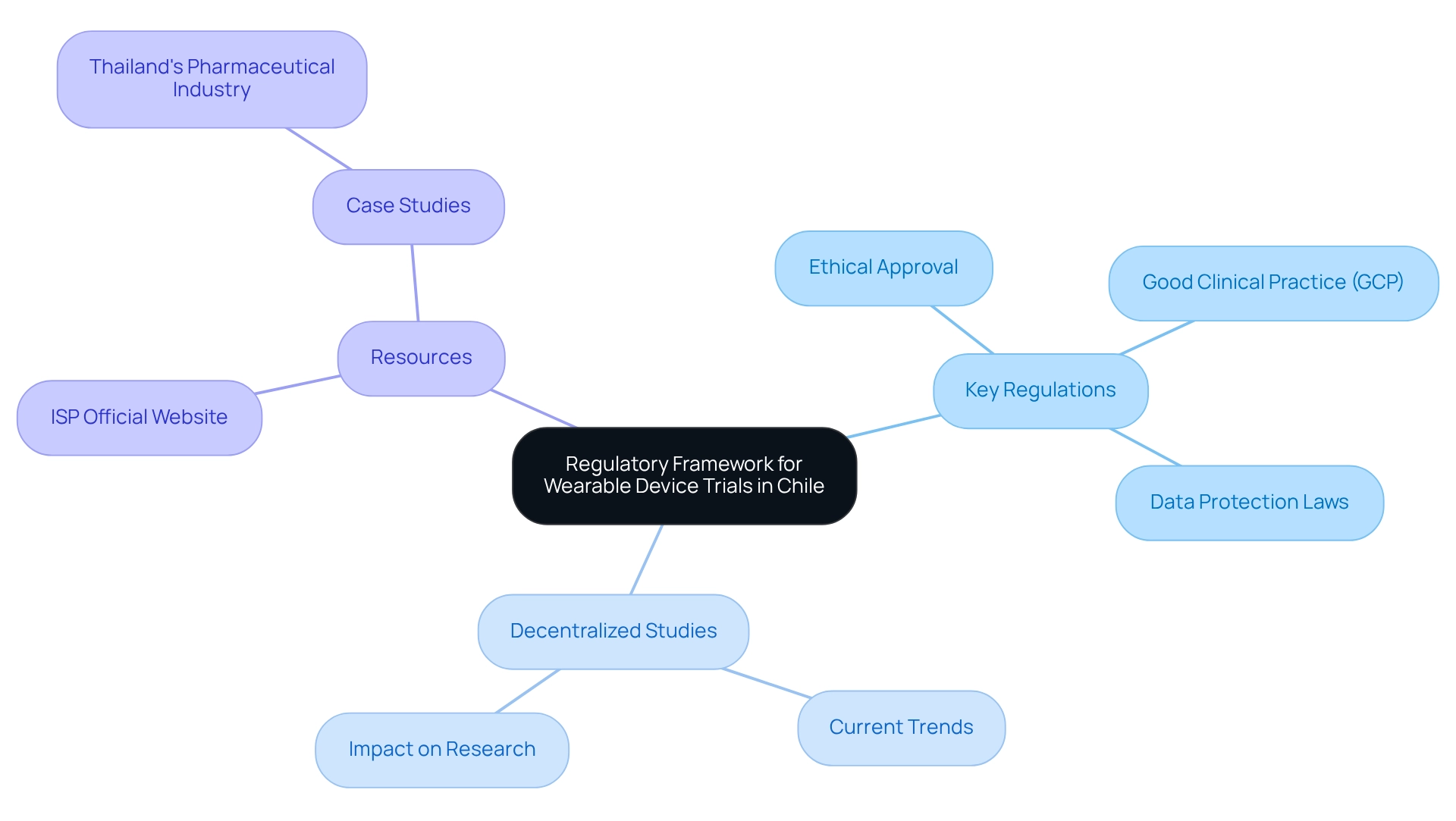
Designing trials for wearable devices in Chile necessitates a thorough understanding of the local regulatory framework. This entails familiarizing oneself with the guidelines set forth by the Chilean Public Health Institute (ISP) and other relevant authorities. Key regulations require:
- Ethical approval
- Adherence to Good Clinical Practice (GCP)
- Compliance with data protection laws
As highlighted by the bioaccess® content team, "The dedication to stringent standards and ethical practices positions Chile as a competitive participant in the global medical equipment arena, paving the way for future innovations that can significantly enhance healthcare delivery." To ensure that your study design aligns with current standards, it is imperative to stay informed about the latest regulations pertaining to medical devices in Chile.
Moreover, with 89% of sponsors now utilizing decentralized studies (DCTs) to support at least one of their projects, it is vital to consider how the regulatory framework is evolving to accommodate these contemporary methodologies. Valuable resources, such as the ISP's official website and recent publications on clinical research regulations in Chile, provide essential insights into the shifting landscape of clinical studies. Furthermore, analyzing case studies from diverse regions, like Thailand's pharmaceutical sector, illustrates the global impact of regulatory compliance and innovative clinical research.
Leveraging the expertise of bioaccess®, which specializes in comprehensive clinical study management services—including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies—can significantly enhance the success of your studies while ensuring compliance and safeguarding information.
Identify Key Regulatory Bodies and Their Roles
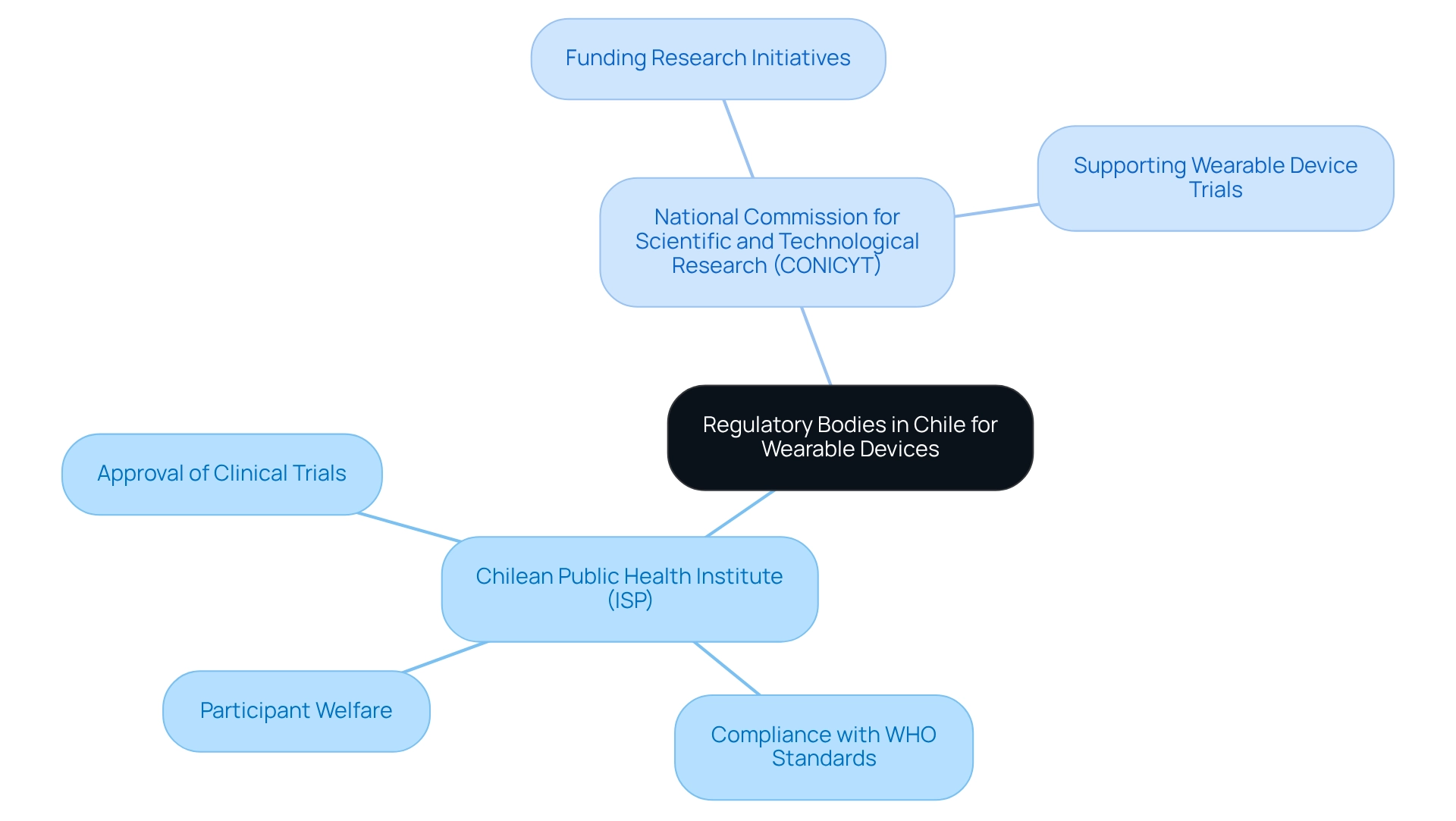
In Chile, the design of trials for wearable devices is primarily governed by the regulatory framework established by the Chilean Public Health Institute (ISP). The ISP is tasked with the approval of clinical trials, which encompasses the design of trials for wearable devices, ensuring compliance with health regulations that protect participant welfare and maintain data integrity.
As highlighted by the bioaccess® content team, "The nation's compliance with global standards and its efficient approval processes make it an attractive location for healthcare assessments, contributing to the global advancement of scientific research and patient care."
Furthermore, the National Commission for Scientific and Technological Research (CONICYT) plays an essential role in funding and supporting research initiatives, particularly in the realm of wearable device trials. Understanding the functions of these organizations, alongside relevant regional health authorities, is crucial for effectively navigating the regulatory landscape in Chile.
The ISP ensures adherence to international standards set by the World Health Organization (WHO) and the International Conference on Harmonisation (ICH), underscoring the robustness of the regulatory framework. Engaging early with these entities can significantly streamline the approval process and enhance compliance, facilitating the successful execution of clinical studies, especially in the context of wearable devices.
Additionally, bioaccess® offers a comprehensive suite of services that includes study set-up, start-up, feasibility assessments, investigator selection, and project management, ensuring that all regulatory requirements are met efficiently.
Moving forward, it is advisable for researchers to foster relationships with the ISP and CONICYT to ensure a seamless testing process. Once these connections are established, researchers should leverage bioaccess®'s commitment to information protection and transparency to address client concerns effectively.
Address Ethical Considerations in Trial Design
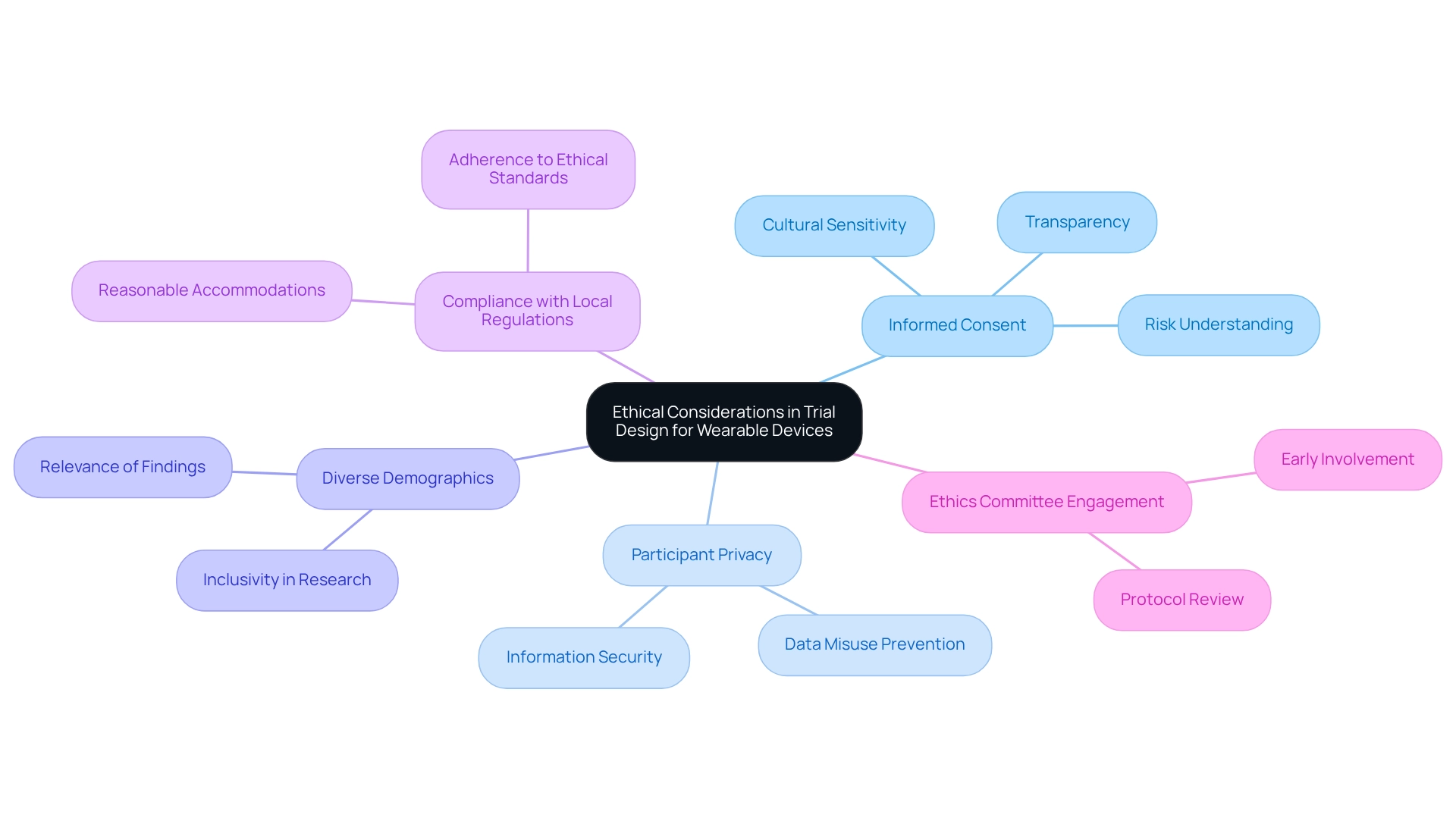
Designing trials for wearable devices in Chile requires a careful approach to ethical considerations, particularly within the diverse landscape of Latin America. Central to this is the informed consent process, which must be transparent and comprehensive, ensuring that participants fully understand the study's purpose, procedures, and potential risks. Adhering to specific informed consent standards that reflect local regulations and cultural sensitivities is essential, as industry leaders emphasize the importance of local expertise in navigating these complexities.
Protecting participant privacy and information security is paramount, especially given the sensitive nature of health information collected through wearable technology. Ethical ramifications extend beyond mere approval; researchers must consider the potential for data misuse and the discomfort participants may experience while using monitoring devices. Engaging with an ethics committee early in the design phase is crucial for reviewing research protocols and ensuring compliance with ethical standards. This proactive approach aligns with market access strategies advocated by experts in the field.
Incorporating diverse participant demographics not only enriches the study but also enhances its ethical integrity, promoting inclusivity and relevance in findings. Case studies underscore the importance of addressing these ethical considerations. For instance, challenges in delivering first-episode psychosis (FEP) services in Chile highlight the necessity of designing trials for wearable devices, alongside evidence-based practices and sufficient training for providers. A recent study indicated that qualitative measures are vital for understanding experiences with FEP services among clients, family members, providers, and policymakers. By learning from such examples, researchers can better navigate the ethical landscape of technology assessments, ultimately leading to improved health outcomes and participant trust.
Moreover, as noted by industry leaders, the commitment to compliance with local regulations, including the provision of reasonable accommodations, is essential for supporting participants throughout the study process. This commitment aligns with the broader shift towards preventive measures in clinical research, emphasizing the significance of ethical considerations in enhancing patient outcomes and ensuring successful market access for innovative medical technologies.
Implement Best Practices for Conducting Wearable Device Trials
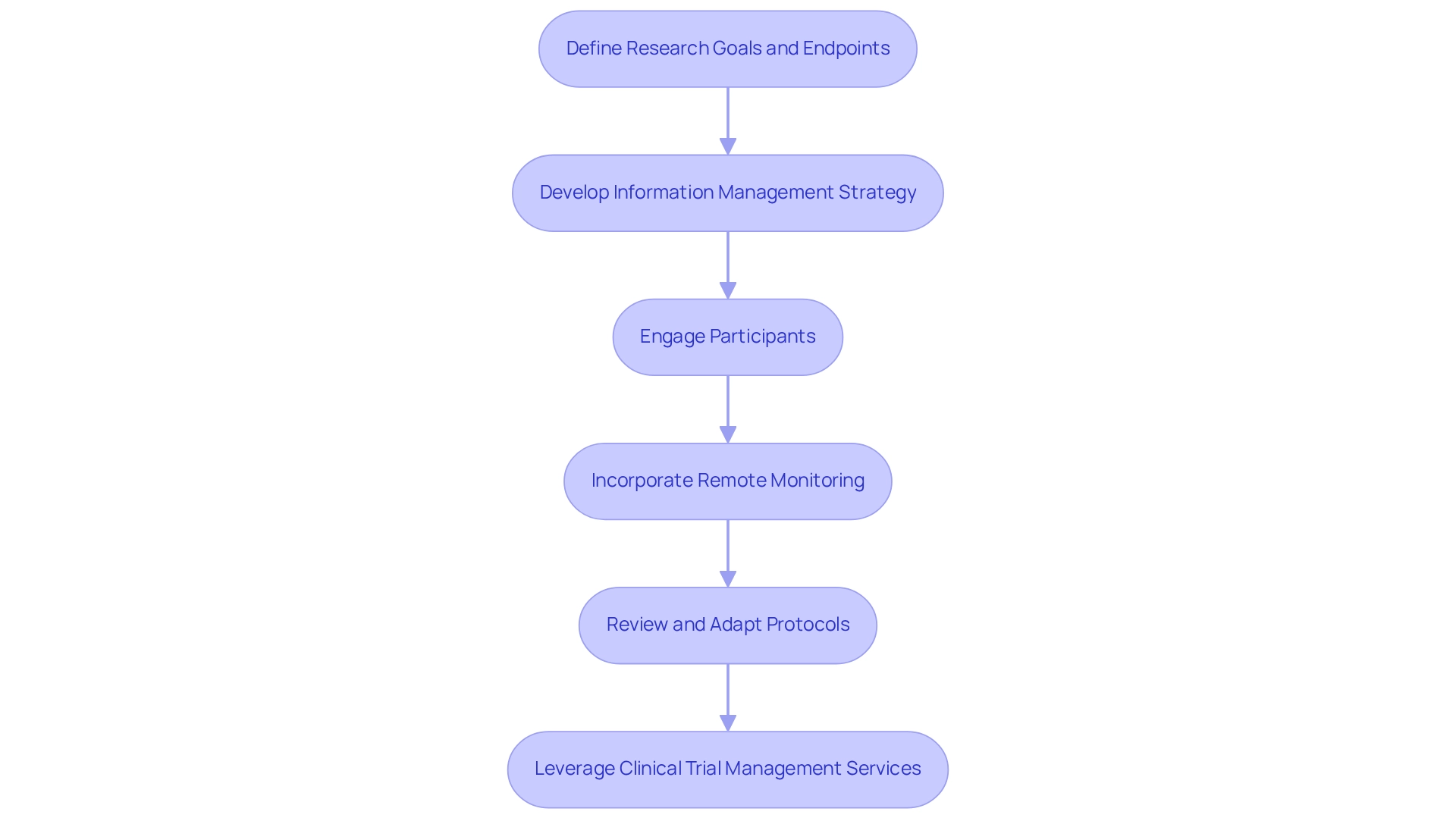
To ensure the success of designing trials for wearable devices in Chile, it is essential to implement best practices throughout the study. Start by choosing endpoints that align with your research goals and are quantifiable through the monitoring technology. This approach is crucial, as designing trials for wearable devices in Chile can showcase the substantial potential of wearables to transform clinical research and emphasize their adoption in various studies.
Create a comprehensive information management strategy that addresses the intricacies of information gathered from devices, encompassing elements of storage, analysis, and reporting. Efficient information management is vital for preserving the integrity and usability of the information, especially considering regulatory compliance factors that necessitate adherence to ethical standards and information protection laws. As highlighted in the case study on regulatory adherence, designing trials for wearable devices in Chile requires ensuring conformity with information protection, patient consent, and adverse event reporting, which is essential for the ethical conduct of clinical trials involving wearable technology.
Engaging participants through regular communication and support is key to addressing any concerns they may have about using the devices. This engagement can significantly improve participant adherence and information accuracy. Incorporating remote monitoring capabilities can further facilitate this process, allowing for real-time data collection and participant feedback. The case study titled 'Wearables in Health Research' underscores the importance of participant training and pilot studies, demonstrating how these elements can greatly enhance the process of designing trials for wearable devices in Chile.
Additionally, it is important to regularly review and adapt trial protocols based on interim findings. This iterative method not only enhances results but also guarantees that the research remains adaptable to the evolving landscape of wearable technology. As Dr. Glimcher aptly stated, "The best gadget is non-invasive," emphasizing the significance of creating tools that prioritize user comfort and ease of use.
By adhering to these optimal methods, researchers can efficiently manage the intricacies involved in designing trials for wearable devices in Chile, ultimately resulting in more dependable and influential outcomes. The evolution of wearable technology is further underscored by the FDA's approval of the first pill containing a smart sensor in 2017, marking a significant milestone in the integration of technology into clinical research. Furthermore, leveraging comprehensive clinical trial management services, such as those offered by bioaccess, can enhance feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting processes, ensuring a successful trial outcome.
Conclusion
Conducting clinical trials for wearable devices in Chile necessitates a multifaceted approach that intertwines a deep understanding of the regulatory landscape, ethical considerations, and best practices in trial design. A comprehensive grasp of the regulations established by the Chilean Public Health Institute and other pivotal bodies is vital for ensuring compliance and safeguarding participant welfare. Early engagement with these organizations can facilitate smoother approvals and significantly enhance the overall success of trials.
Ethical considerations are paramount in the design of wearable device studies. Transparent informed consent processes, robust data protection measures, and inclusivity in participant demographics not only uphold ethical standards but also cultivate trust and integrity in research. By drawing lessons from past case studies and actively involving ethics committees, researchers can adeptly navigate the complexities of trial implementation while prioritizing participant safety and comfort.
Implementing best practices throughout the trial process is crucial for maximizing the effectiveness of wearable technology in clinical research. From selecting appropriate endpoints to establishing comprehensive data management plans, each step is instrumental in ensuring the reliability and impact of the results. Continuous engagement with participants and adaptability in trial protocols further enhance the study's responsiveness to emerging challenges and innovations.
In conclusion, the successful execution of wearable device trials in Chile hinges on a thorough understanding of regulatory frameworks, a steadfast commitment to ethical standards, and the diligent application of best practices. As the demand for innovative healthcare solutions continues to surge, these efforts not only propel scientific inquiry forward but also enhance healthcare delivery, paving the way for a future where technology and patient care are seamlessly integrated.
Frequently Asked Questions
What is necessary for designing trials for wearable devices in Chile?
Designing trials for wearable devices in Chile requires a thorough understanding of the local regulatory framework, including familiarization with guidelines set by the Chilean Public Health Institute (ISP) and other relevant authorities.
What are the key regulations for conducting clinical trials in Chile?
Key regulations include obtaining ethical approval, adhering to Good Clinical Practice (GCP), and complying with data protection laws.
Why is Chile considered a competitive participant in the global medical equipment arena?
Chile is viewed as competitive due to its dedication to stringent standards and ethical practices, which pave the way for future innovations that can significantly enhance healthcare delivery.
How can researchers stay informed about the latest regulations for medical devices in Chile?
Researchers can stay informed by consulting valuable resources such as the ISP's official website and recent publications on clinical research regulations in Chile.
What percentage of sponsors are now utilizing decentralized studies (DCTs)?
Currently, 89% of sponsors are utilizing decentralized studies (DCTs) to support at least one of their projects.
What role does bioaccess® play in clinical study management?
Bioaccess® specializes in comprehensive clinical study management services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, which can enhance the success of studies while ensuring compliance and safeguarding information.
How can case studies from other regions, like Thailand, inform research in Chile?
Analyzing case studies from regions like Thailand illustrates the global impact of regulatory compliance and innovative clinical research, providing insights that can be beneficial for conducting trials in Chile.




