Overview
The article highlights the emerging significance of Latin America as a prime location for medical trial recruitment, emphasizing its diverse patient demographics, cost advantages, and improved regulatory frameworks. It asserts that effective recruitment strategies are crucial, particularly those that address cultural nuances and socioeconomic barriers. These strategies are essential for enhancing participation rates and ensuring the success of clinical trials in the region.
By recognizing the distinct characteristics of Latin American populations, researchers can tailor their approaches to foster greater engagement and participation. This strategic focus not only improves trial outcomes but also contributes to the advancement of clinical research in a rapidly evolving landscape.
Introduction
As the landscape of clinical trials continues to evolve, Latin America has emerged as a significant player, attracting attention from Medtech companies worldwide. With its rich diversity in patient demographics, cost advantages, and improving regulatory frameworks, countries like Brazil and Mexico present unique opportunities for conducting impactful clinical research.
However, the region also faces challenges in:
- Recruiting participants
- Navigating complex regulations
- Ensuring equitable representation in trials
By understanding these dynamics and implementing effective strategies, stakeholders can harness the potential of Latin America to foster innovation and enhance healthcare outcomes. This article delves into the intricacies of clinical trials in this vibrant region, exploring the opportunities and challenges that define its role in the global clinical research landscape.
The Emergence of Latin America as a Prime Location for Clinical Trials
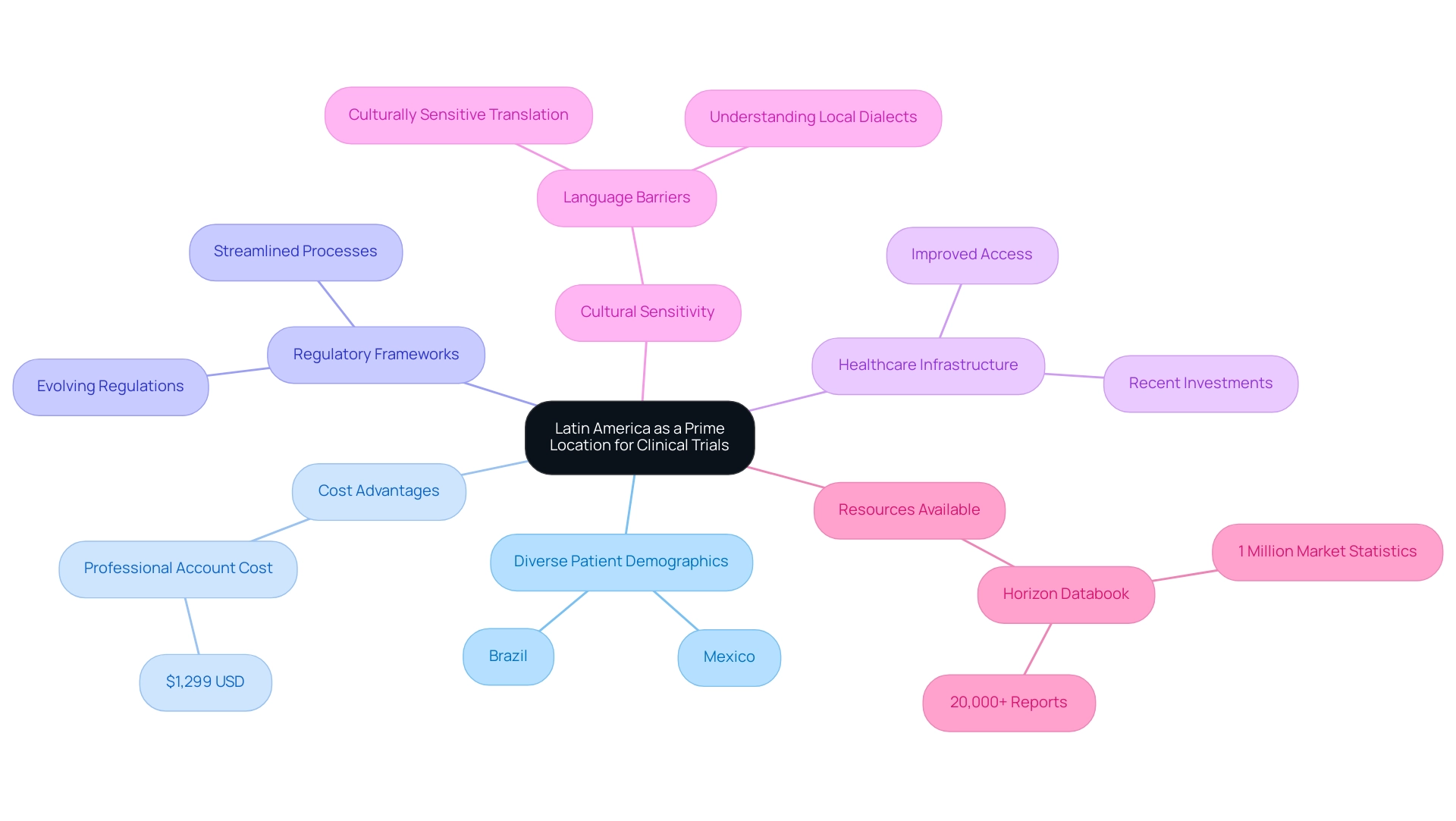
Latin nations have emerged as a pivotal area for medical trial recruitment in Latin America, propelled by their diverse patient demographics, significant cost advantages, and evolving regulatory frameworks. The extensive and varied participant pool provided by Brazil and Mexico is essential for medical trial recruitment in Latin America, enhancing the generalizability of research outcomes. The urbanized populations within these countries, coupled with a spectrum of health challenges, present unique opportunities for Medtech firms to conduct studies that are both effective and meaningful.
Recent investments in healthcare infrastructure and regulatory reforms have streamlined the process for international sponsors, facilitating smoother navigation of the research landscape in Latin regions. This evolution not only accelerates the timeline for introducing innovative medical devices to the market but also ensures that evaluations are conducted in an environment attuned to local health needs. Furthermore, the increasing number of studies in Latin America underscores the region's growing attractiveness for medical trial recruitment.
Effective medical trial recruitment in Latin America, particularly in Brazil and Mexico, leverages local knowledge and cultural nuances, which are crucial for fostering participant trust and engagement. For instance, addressing language barriers through culturally sensitive translation methods has proven essential in enhancing informed consent processes, as highlighted in the case study on language obstacles and translation in medical studies. This approach minimizes misunderstandings and ultimately improves the overall quality of medical research.
As the global market for medical research continues to evolve, Latin America emerges as a compelling option for Medtech companies aiming to engage in medical trial recruitment. This region not only offers cost-effectiveness—evidenced by the Professional Account for teams costing $1,299 USD—but also boasts a wealth of diversity and relevance. With over 20 years of expertise in the Medtech sector, bioaccess® delivers expedited medical device research services, concentrating on Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies. Our customized approach ensures comprehensive management throughout the process, adeptly navigating regulatory challenges and enhancing operational efficiency.
While projections indicate that North regions will dominate the global market by 2030, the strategic positioning of Latin territories as an emerging hub for medical trial recruitment presents a unique opportunity for stakeholders in the Medtech industry. Additionally, resources such as the Horizon Databook, which provides access to over 1 million market statistics and more than 20,000 reports, offer invaluable insights into market trends, customer preferences, and competitor strategies, further reinforcing the rationale for conducting studies in this region.
Challenges in Clinical Trial Recruitment in Latin America
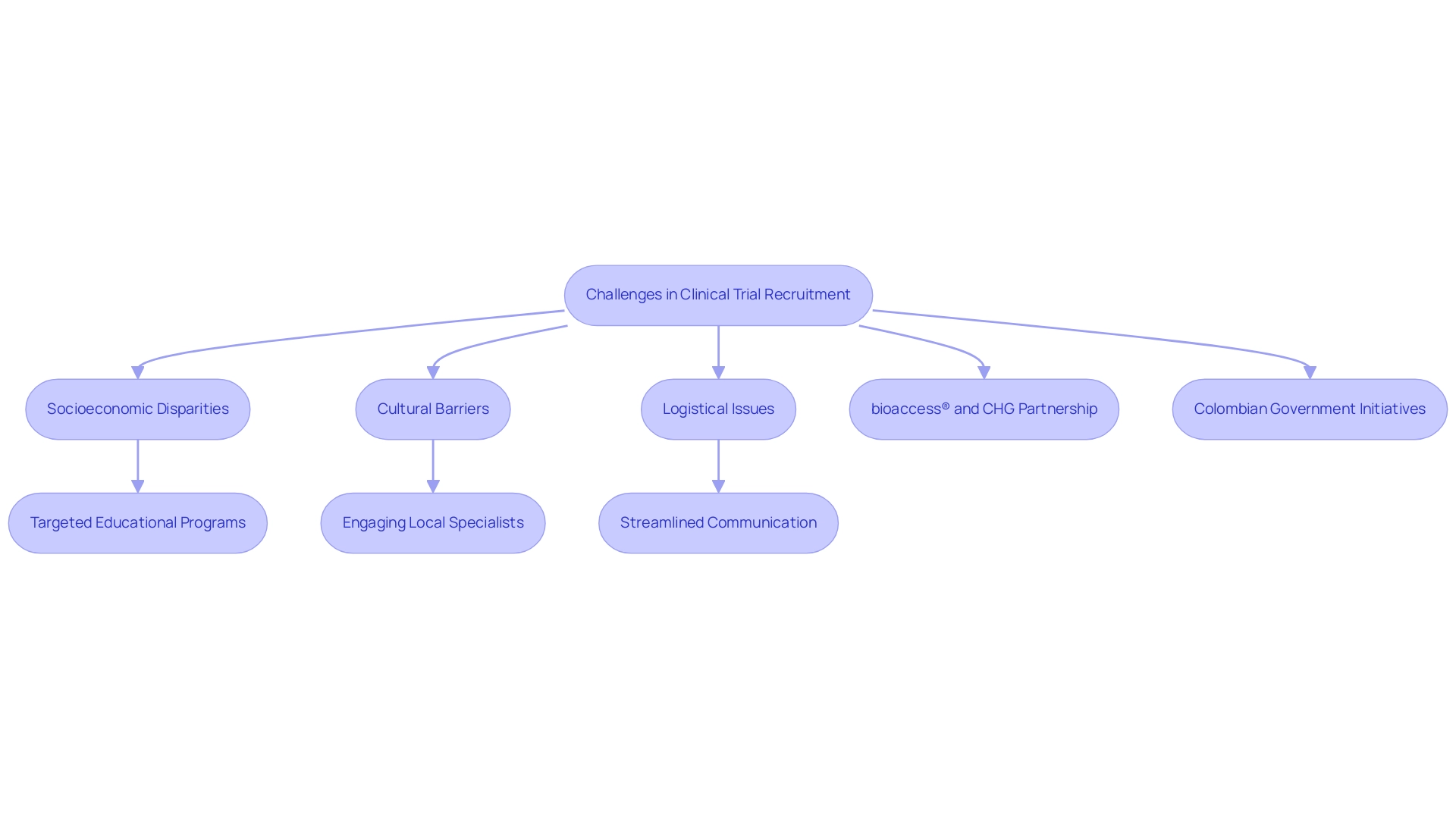
Medical trial recruitment in Latin America encounters significant obstacles that critically influence participation rates. Socioeconomic disparities are crucial, as individuals from lower-income backgrounds often face barriers such as limited access to healthcare facilities and insufficient transportation options. These logistical challenges can deter potential participants from engaging in medical studies, ultimately affecting the diversity and representativeness of participant groups.
Cultural barriers further complicate recruitment efforts. Many individuals harbor misconceptions about the safety and efficacy of research studies, leading to reluctance in participation. This lack of awareness regarding the benefits and procedures associated with medical research underscores the need for targeted educational programs that elucidate research studies and foster trust within local communities.
A case study titled "Understanding Patient Diversity in Latin regions" emphasizes the importance of patient diversity and demonstrates how addressing these challenges can enhance healthcare outcomes. By catering to the unique needs of various groups, research studies can be designed to be more inclusive, ultimately resulting in tailored healthcare solutions that better serve the region.
Moreover, effective strategies for conducting medical studies in Latin regions highlight the importance of engaging local specialists and maintaining cultural sensitivity throughout the recruitment process. Streamlined communication and comprehensive training for research teams can further bolster recruitment initiatives, facilitating a more effective approach to overcoming the socioeconomic barriers that impede participation. As noted by Julio G. Martinez-Clark, CEO of bioaccess®, "The government of Colombia appears to be the sole nation in Latin regions with a proactive initiative to draw in more medical studies as part of its strategy to evolve into a knowledge-based economy by 2031."
This perspective underscores the regional initiatives aimed at enhancing medical trial recruitment in Latin America, thereby improving participation in research studies and recruitment strategies.
In collaboration with Caribbean Health Group (CHG), bioaccess™ is actively working to establish Barranquilla as a leading hub for medical trial recruitment in Latin America, with the support of the Colombian Minister of Health. With over 15 years of experience in the Medtech industry, bioaccess® specializes in managing various types of studies, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
This partnership aims to enhance medical study ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and 95% retention rates.
As the landscape of medical research evolves, recognizing and addressing these challenges will be essential for advancing healthcare technology and ensuring equitable healthcare solutions throughout Latin regions.
Effective Strategies for Patient Recruitment in Latin American Trials
To enhance medical trial recruitment in Latin America, it is essential to implement community-focused strategies that actively involve local populations in research studies. Collaborating with local healthcare providers can significantly improve outreach efforts, while social media platforms serve as effective tools for disseminating information and attracting potential participants. Informational sessions that educate communities about the advantages and safety of research studies can further demystify the process and encourage participation.
Tailoring communication to respect cultural nuances and address language barriers is crucial. Employing bilingual staff and providing culturally relevant materials can greatly enhance trust and participation among diverse groups. Additionally, leveraging patient advocacy groups not only facilitates outreach but also fosters a sense of community ownership in the research process.
This collaborative strategy can lead to improved retention rates and more successful medical studies, as demonstrated by case analyses showcasing the positive influence of strategic communication plans that prioritize patient needs. Notably, the maximum number of retention tactics overall was 10 out of a possible 12 listed, underscoring the importance of a robust strategy.
Furthermore, bioaccess® provides extensive research study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting. This positions bioaccess as a prominent contract research organization supporting medical trial recruitment in Latin America, ensuring that studies are conducted effectively and in accordance with local regulations.
The case study titled 'Understanding the Patient's Needs' illustrates that high dropout rates in research studies can arise from misunderstandings regarding expectations and personal issues faced by patients. A strong communication plan is essential for addressing these challenges. As Scott Schliebner, Vice President and Global Head of Drug Development Consulting, observed, by 2025, medical studies will experience transformative advancements propelled by innovation, patient-centricity, and regulatory evolution.
Moreover, the recent shift towards greater utilization of digital tools and online approaches for recruitment and retention in research after the pandemic underscores the importance of adapting strategies to contemporary trends. Specific examples from bioaccess's recent studies demonstrate how tailored communication and community engagement have led to increased participant retention and satisfaction.
Navigating the Regulatory Framework for Clinical Trials in Latin America
Navigating the regulatory landscape for medical trial recruitment in Latin America demands a comprehensive understanding of the unique requirements established by each country. As of 2025, regulatory bodies such as ANVISA in Brazil and COFEPRIS in Mexico have refined their guidelines, which are essential for obtaining trial approvals. For example, the submission process for multicenter study protocols in Brazil necessitates that the coordinating center's ethics committee (EC) reviews the protocol prior to its submission to CONEP for further evaluation.
This organized approach not only enhances collaboration among participating institutions but also ensures consistency in ethical oversight across various study sites.
In Colombia, bioaccess offers extensive research study management services that include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project management
- Comprehensive reporting on study status, inventory, and adverse events
These services are tailored to streamline the approval process and ensure adherence to ethical standards, which is crucial for sponsors and contract research organizations (CROs). Colombia's competitive advantages, such as over 30% cost reductions compared to North America and Western Europe, a rapid IRB/EC and MoH (INVIMA) review process lasting only 90-120 days, and a high-quality healthcare system recognized globally, position it as an attractive location for medical trial recruitment in Latin America.
Staying informed about these evolving regulations is essential for sponsors and CROs. Engaging local regulatory experts, such as Katherine Ruiz, who specializes in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, can significantly expedite the approval process and ensure compliance with ethical standards. Furthermore, understanding the nuances of local regulations is vital for designing studies that are both compliant and culturally sensitive, thereby enhancing medical trial recruitment in Latin America and improving participant retention.
In Mexico, COFEPRIS guidelines for research studies have also been updated, underscoring the need for sponsors to provide detailed descriptions of actions taken to ensure adherence to good practices, particularly for investigational new drug (IND) submissions that include data from studies conducted outside the U.S. Notably, the sponsor must submit an original and two copies for paper IND submissions, highlighting the importance of meticulous planning and local insight in effectively navigating the regulatory framework.
Moreover, the processing of personal data of children and adolescents must prioritize their best interests and require consent from a parent or guardian, emphasizing the ethical responsibilities inherent in clinical research.
As Alexandre Dalmasso, a partner at Licks Attorneys, observes, "Navigating the regulatory landscape requires not only compliance but also a deep understanding of local contexts and ethical considerations." This perspective underscores the importance of leveraging expert insights on regulatory frameworks for the successful execution of medical trial recruitment in Latin America. By prioritizing compliance and cultural considerations, organizations can significantly enhance their chances of achieving timely and successful approvals.
Promoting Diversity and Inclusion in Clinical Trial Participation
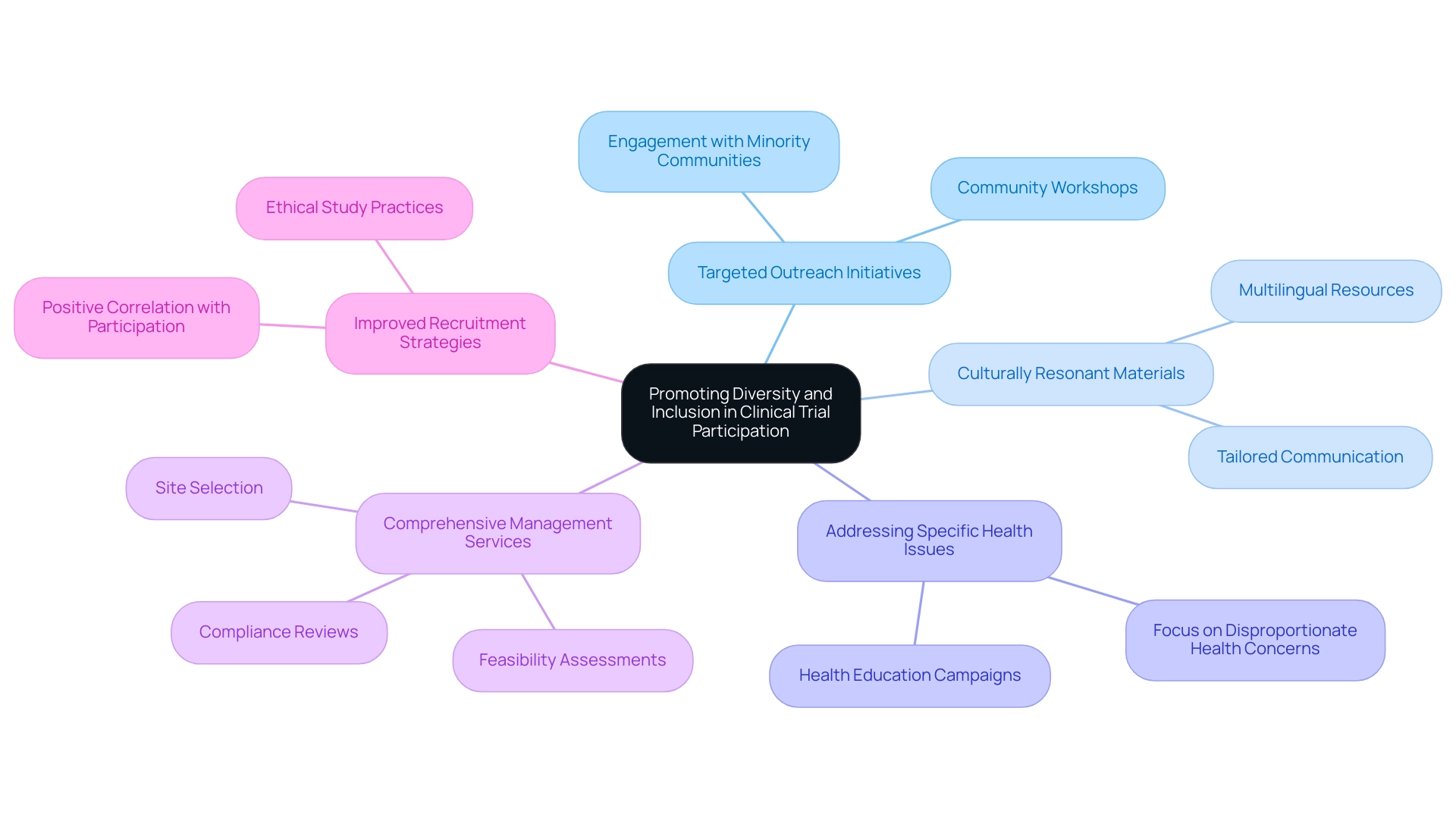
Encouraging diversity and inclusion in study participation is crucial for ensuring that findings accurately represent the wider population. In Latin America, renowned for its diverse ethnic and cultural backgrounds, the recruitment of underrepresented groups in health studies is particularly significant. As Sonia S Anand, Canada research chair in ethnic diversity and cardiovascular disease, asserts, "Researchers should strive to achieve proportional representation by ethnicity in their studies as reflected by disease burden or the census."
Effective strategies to enhance diversity encompass:
- Targeted outreach initiatives aimed at minority communities
- Ensuring that materials resonate culturally
- Addressing specific health issues that disproportionately affect these populations
With over 15 years of experience in the Medtech sector, bioaccess® understands the complexities of conducting research studies in this diverse environment. The recent partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a premier location for medical trial recruitment in Latin America, supported by Colombia's Minister of Health.
This collaboration not only enhances the feasibility of conducting various research studies, including Early-Feasibility Studies and First-In-Human Studies, but also underscores the importance of comprehensive management services for medical trial recruitment in Latin America. These services include:
- Feasibility assessments
- Site selection
- Compliance reviews
Furthermore, a study titled "Limitations and Considerations in Clinical Trial Participation Studies" highlights a positive correlation between stated willingness to participate and actual participation, emphasizing the necessity for improved recruitment strategies. By fostering an inclusive environment, researchers can significantly boost recruitment rates, thereby enhancing the validity and applicability of their findings.
This approach not only adheres to ethical study practices but also elevates the overall quality of medical investigations, ultimately leading to better health outcomes for all communities involved. Notably, the partnership has achieved more than a 50% reduction in recruitment duration and 95% retention rates, demonstrating its effectiveness in enhancing research participation.
The Role of CROs in Enhancing Clinical Trial Recruitment and Execution
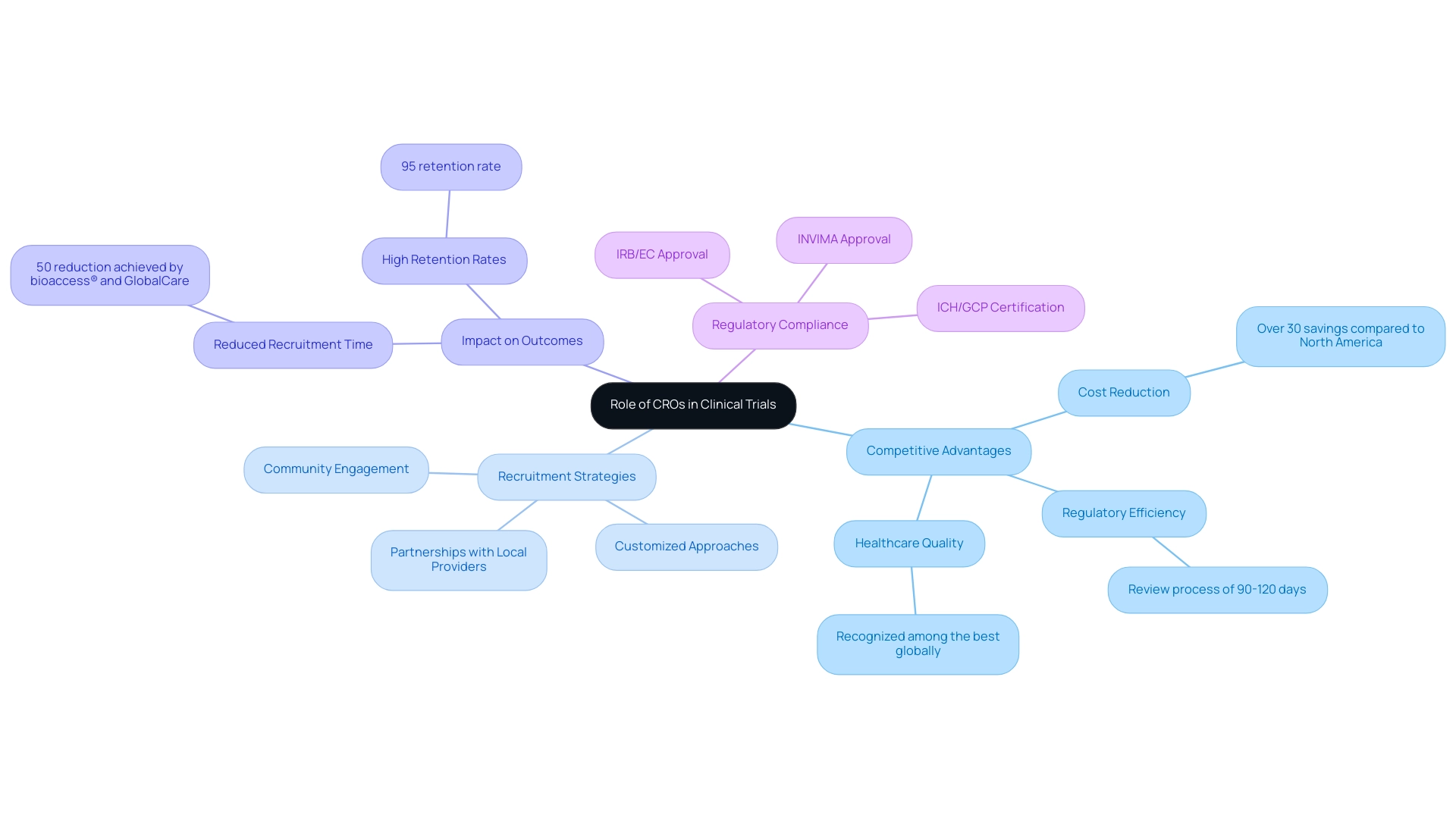
Contract research organizations (CROs) play a pivotal role in enhancing medical trial recruitment in Latin America and executing clinical studies, a region that presents an unmatched opportunity for cost-effective and impactful clinical research. Colombia stands out due to its competitive advantages, including:
- A cost reduction exceeding 30% compared to studies conducted in North America or Western Europe.
- A regulatory review process that spans only 90-120 days.
- A healthcare system recognized among the best globally.
These elements significantly bolster the feasibility of conducting first-in-human (FIH) studies.
CROs possess an in-depth understanding of local healthcare dynamics and established networks that empower them to expedite patient recruitment while streamlining study processes effectively. By skillfully navigating the intricate regulatory landscape, which includes securing IRB/EC approval and INVIMA approval, CROs ensure compliance and manage logistics, significantly alleviating the operational burden on sponsors.
Innovative recruitment strategies are a hallmark of CROs, who customize their approaches to resonate with local communities. Initiatives such as community engagement and partnerships with local healthcare providers not only enhance recruitment efficiency but also cultivate trust and participation among potential study subjects. For instance, the collaboration between bioaccess®, a leading CRO in Latin America, and GlobalCare Clinical Trials in Colombia exemplifies this strategy, achieving over a 50% reduction in recruitment time and an impressive 95% retention rate.
Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, shared his positive experience with bioaccess during its initial human study in Colombia, underscoring the effectiveness of CROs in enhancing research outcomes.
Moreover, Colombia provides substantial R&D tax incentives, including a 100% tax deduction for investments in science, technology, and innovation projects, further elevating its appeal for clinical trials. As the demand for improved healthcare access and diversity in enrollment grows, the role of CROs in medical trial recruitment in Latin America becomes increasingly vital. As noted by Allison Kalloo, "The current landscape is influenced by a demand for enhanced healthcare access and a commitment to diversity in participant enrollment."
Their ability to leverage real-world data (RWD) and real-world evidence (RWE) is crucial in informing regulatory and healthcare decision-making, thereby enhancing medical trial recruitment in Latin America and positioning them as key contributors in the evolving landscape of medical inquiry. Furthermore, hospitals in Colombia must undergo a rigorous ICH/GCP certification process to conduct research, ensuring adherence to high-quality standards. In 2025, strategic partnerships with CROs will continue to be transformative, driving impactful clinical trials that not only advance medical technology but also improve patient outcomes across the region.
Conclusion
Latin America has emerged as a pivotal hub for clinical trials, propelled by its diverse patient populations, cost advantages, and evolving regulatory landscapes. Countries such as Brazil and Mexico present substantial opportunities for Medtech companies; however, challenges like participant recruitment and cultural barriers remain prevalent.
To bolster recruitment efforts, stakeholders must implement community-focused strategies that engage local populations and foster trust. Collaborating with local healthcare providers and leveraging digital outreach can markedly enhance participation rates. Moreover, promoting diversity and inclusion in clinical trials is essential to ensure that research outcomes accurately reflect the broader population.
Contract research organizations (CROs) are instrumental in navigating the regulatory landscape and refining recruitment strategies. Their expertise can streamline trial processes, ensuring compliance and boosting participant engagement.
In summary, while Latin America offers immense potential for impactful clinical trials, addressing existing challenges is imperative. By nurturing collaboration, embracing diversity, and harnessing the expertise of CROs, stakeholders can propel medical technology forward and enhance healthcare outcomes. This collective endeavor positions Latin America as a crucial player in the global clinical research arena.
Frequently Asked Questions
Why are Latin nations important for medical trial recruitment?
Latin nations, particularly Brazil and Mexico, offer diverse patient demographics, significant cost advantages, and evolving regulatory frameworks, making them pivotal for medical trial recruitment in Latin America.
What advantages do Brazil and Mexico provide for medical trials?
They provide an extensive and varied participant pool, enhancing the generalizability of research outcomes. Their urbanized populations and diverse health challenges present unique opportunities for effective and meaningful studies.
How have recent investments in healthcare infrastructure impacted medical trials in Latin America?
Investments and regulatory reforms have streamlined the process for international sponsors, facilitating smoother navigation of the research landscape, accelerating the introduction of innovative medical devices, and ensuring evaluations meet local health needs.
What role does local knowledge play in medical trial recruitment?
Leveraging local knowledge and cultural nuances is crucial for fostering participant trust and engagement, which can enhance recruitment efforts and improve the quality of medical research.
What challenges does medical trial recruitment face in Latin America?
Recruitment faces significant obstacles such as socioeconomic disparities, limited access to healthcare, transportation issues, and cultural barriers that create misconceptions about the safety and efficacy of research studies.
How can educational programs improve participation in medical trials?
Targeted educational programs can help clarify the benefits and procedures of medical research, fostering trust within local communities and addressing misconceptions that deter participation.
What strategies can enhance medical trial recruitment in Latin America?
Engaging local specialists, maintaining cultural sensitivity, streamlined communication, and comprehensive training for research teams can effectively overcome socioeconomic barriers to participation.
What initiatives are being taken to improve medical trial recruitment in Colombia?
Bioaccess™, in collaboration with Caribbean Health Group and the Colombian Minister of Health, aims to establish Barranquilla as a leading hub for medical trial recruitment, reducing recruitment time and improving retention rates.
What types of studies does bioaccess® specialize in?
Bioaccess® specializes in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies.
What resources are available for understanding market trends in medical research?
The Horizon Databook provides access to over 1 million market statistics and more than 20,000 reports, offering valuable insights into market trends, customer preferences, and competitor strategies.




