Introduction
In the realm of medical device manufacturing, ensuring the safety and effectiveness of sterilization processes is paramount. Sterilization validation serves as a critical framework for confirming that medical devices are free from harmful pathogens, thereby safeguarding patient health and complying with regulatory mandates.
This comprehensive article delves into the systematic steps involved in achieving sterilization validation, explores various sterilization methods with a focus on Ethylene Oxide, and highlights the intricate regulatory landscape that governs these practices.
By examining the essential role of documentation and the importance of adhering to established protocols, this discussion aims to equip professionals in the medical device sector with the knowledge necessary to navigate the complexities of sterilization validation effectively.
Understanding Sterilization Validation for Medical Devices
Sterilization validation for medical devices is a crucial systematic procedure designed to ensure that medical instruments are properly sterilized to eliminate possible pathogens. This confirmation is paramount for maintaining patient safety and adhering to stringent regulatory standards. The procedure involves rigorous testing and thorough documentation to ensure the sterilization validation for medical devices, confirming that disinfection methods consistently meet the desired sterility assurance level (SAL).
According to FDA guidelines, 510(k) holders are advised to document all qualification activities related to decontamination site changes within their internal files, reinforcing the importance of meticulous record-keeping in the assessment. Furthermore, it is essential for experts in the medical device industry to understand the concepts behind the process of ensuring cleanliness, as these concepts greatly impact product quality and adherence to regulatory standards. Recent insights indicate that the chance of qualification decreases as sample size increases, with specific probabilities of qualification provided for different sample sizes and failure rates, highlighting the importance of statistical frameworks in validating decontamination methods.
As Harriet B. Braiker, MD, states, "Striving for excellence motivates you; striving for perfection is demoralizing," highlighting the necessity for a balanced method in achieving high standards in process verification. Moreover, the EtO Master File Pilot Program for PMA holders acts as a real-world illustration of how the process of ensuring safety influences regulatory procedures, easing the submission requirements for Class III medical device producers. Overall, a strong grasp of sterilization validation for medical devices not only impacts regulatory adherence but is also essential for ensuring the highest levels of patient safety.
Step-by-Step Process for Achieving Sterilization Validation
- Define the Sterilization Validation for Medical Devices: Begin by identifying the sterilization method that requires validation for medical devices. Common methods include Ethylene Oxide, steam, and radiation, each with distinct parameters and applications.
- Establish Verification Protocols: Develop a comprehensive verification protocol that articulates the objectives, methodology, and acceptance criteria. This framework is crucial for ensuring that the verification process is systematic and reproducible.
- Perform Pre-Validation Studies: Conduct preliminary studies to evaluate the effectiveness of the selected sterilization method. These studies assist in recognizing any potential challenges before moving on to full-scale confirmation.
- Execute the Verification Study: Implement the verification study in accordance with the established protocol. It is essential to monitor and document all parameters meticulously to ensure data integrity and reliability. Additionally, record the consumption of sanitation cycle consumables to ensure comprehensive documentation and resource management.
- Analyze Results: After the validation study, review the collected data to ascertain whether the disinfection method meets the predetermined criteria. Statistical analysis may be necessary to evaluate the success rates of various methods for preventing pregnancy, particularly as advancements continue to emerge in 2024. It's important to note that the incidence of Post Ablation Tubal Sterilization Syndrome is estimated to be approximately 10% to 20%, highlighting the significance of sterilization validation for medical devices and its implications for patient outcomes.
- Compile Documentation: Prepare detailed reports that summarize the assessment, results, and conclusions. This documentation is vital for regulatory compliance and can serve as a reference for future validations.
- Implement Routine Monitoring: Establish ongoing monitoring protocols to ensure that the disinfection method remains compliant and effective over time. Regular audits and reviews are essential to adapt to any changes in standards or technology, thus maintaining the highest quality of sanitation practices. Insights from case studies, like the 'Nursing and Interprofessional Team Interventions,' highlight the essential role of teamwork and adherence to protocols in the sanitation procedures, ensuring successful surgical outcomes.
Exploring Different Sterilization Methods: Focus on Ethylene Oxide
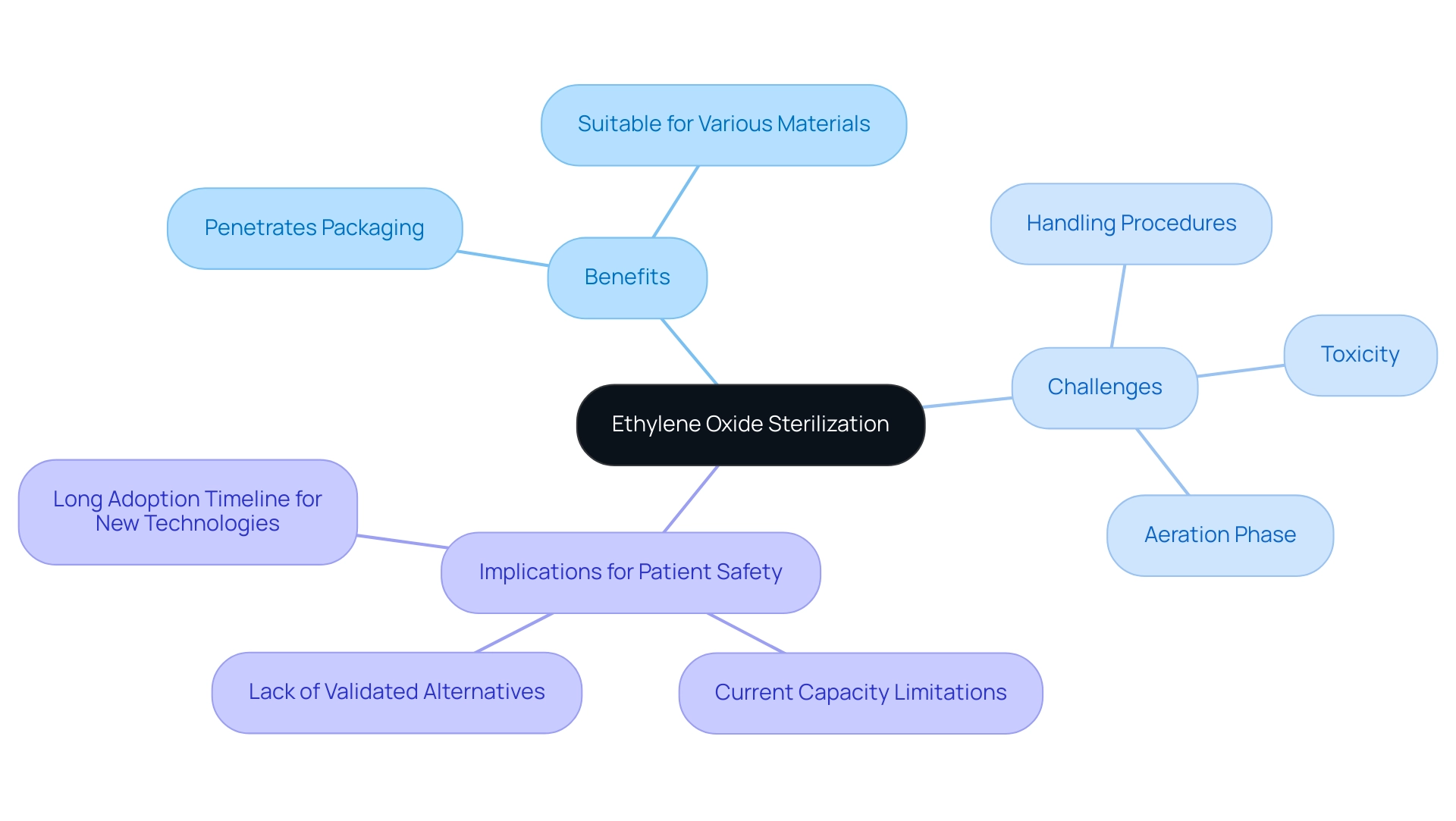
Ethylene Oxide (EtO) purification serves as an essential technique for guaranteeing the safety of heat-sensitive medical instruments. This process involves exposing items to EtO gas, which effectively penetrates packaging and neutralizes a wide range of microorganisms, making it particularly beneficial for sterilizing complex instruments. Among its benefits, EtO disinfection is suitable for various materials, enabling a wider use across medical instruments.
However, the use of EtO is not without its challenges. The toxic nature of the gas necessitates meticulous handling procedures, and a crucial post-sterilization phase—known as aeration—is required to eliminate residual EtO before the devices can be safely used. The operational challenges linked to novel decontamination technologies emphasize the significance of comprehending the specific requirements and processes involved in sterilization validation for medical devices, particularly in relation to EtO treatment for successful validation.
As noted in the case study titled 'Operational Challenges of Novel Disinfection Technologies,' these novel methods face limitations that may hinder their industrial-scale adoption, with current EtO facilities operating at maximum capacity and no validated alternatives available. This poses serious implications for patient care and safety. Furthermore, with very few single-use medical devices (only 5) validated for chlorine dioxide disinfection, the reliance on EtO remains critical amidst current safety regulations.
The benefits and drawbacks of various technologies for disinfection, including EtO, must be carefully considered, as highlighted in recent discussions on the disinfection landscape. As Hippocrates aptly stated,
Primum non nocere <—“first do no harm”—this principle must be at the forefront of our approach to disinfection, ensuring that patient safety remains paramount.
Navigating Regulatory Requirements for Sterilization Validation
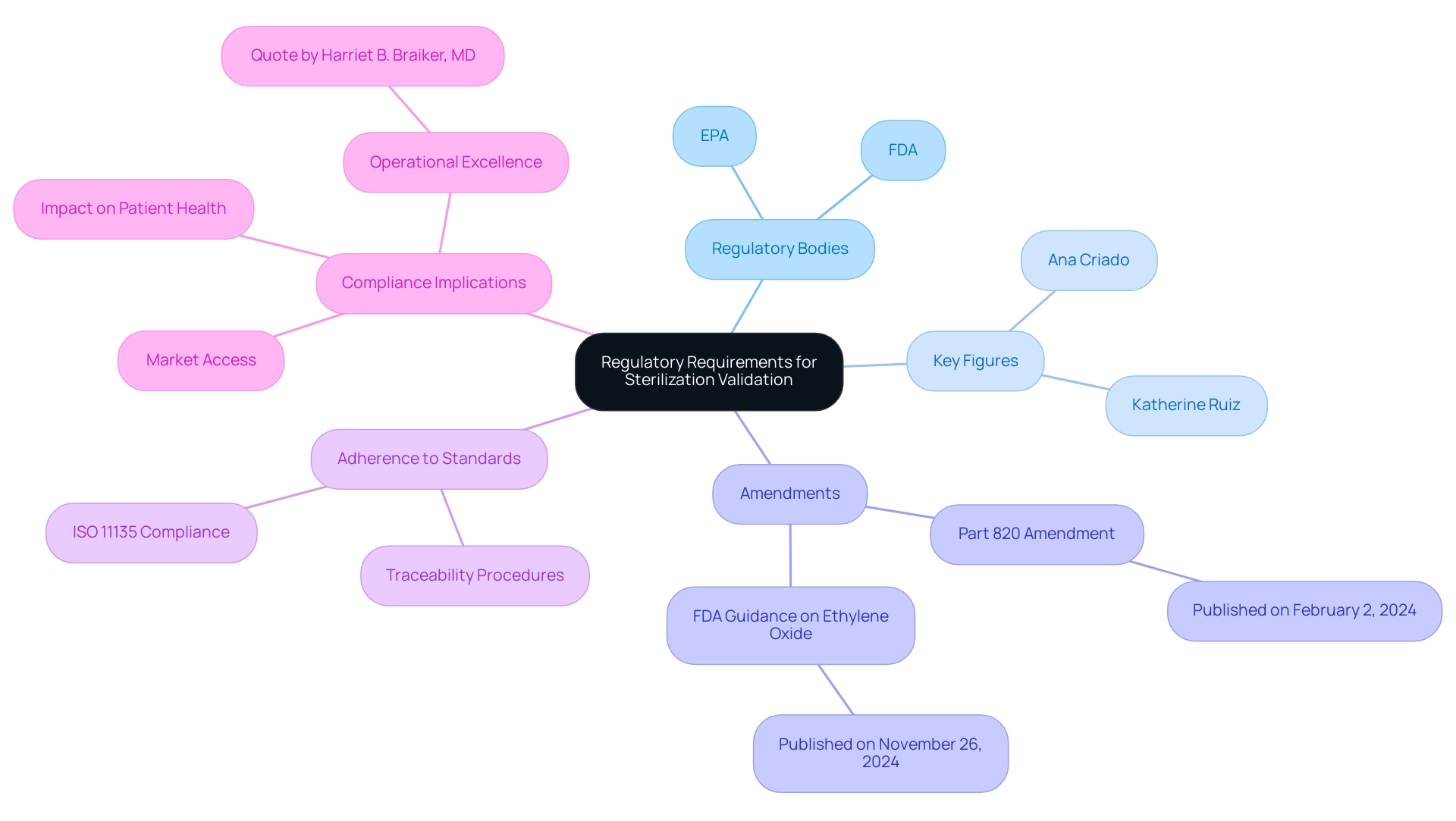
Regulatory requirements for sterilization validation for medical devices depend on the guidelines established by the FDA and EPA, requiring that manufacturers validate the effectiveness of their disinfection methods through rigorous studies and meticulous documentation. Key figures, such as Ana Criado, Director of Regulatory Affairs and Professor, who has extensive experience in regulatory consulting and biomedical engineering, emphasize the importance of these standards. A recent amendment to Part 820, published on February 2, 2024, emphasizes the necessity for manufacturers to establish procedures for traceability, ensuring that each unit or batch is identifiable throughout the production of surgical implants and life support devices.
Adherence to ISO 11135 for ethylene oxide (EtO) disinfection is particularly vital for sterilization validation for medical devices, as it offers a structure for guaranteeing that disinfection practices meet rigorous safety and legal standards. Currently, each manufacturer must establish and maintain procedures for identifying products throughout all stages—from receipt and production to distribution and installation. This comprehensive approach not only protects patient health but also secures market access.
Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, reinforces the importance of adhering to these practices. The FDA's recent guidance published on November 26, 2024, regarding changes in ethylene oxide disinfection activities allows certain disinfection facility changes without prior approval, streamlining the process for affected manufacturers. Moreover, statistics suggest that compliance rates with ISO 11135 for sterilization validation for medical devices through EtO processing are essential, with recent data demonstrating that adherence rates have enhanced considerably, ensuring consistent quality and safety in medical equipment.
As articulated by Harriet B. Braiker, MD, 'Striving for excellence motivates you; striving for perfection is demoralizing,' it is imperative for organizations in the medical device field to focus on operational excellence and compliance to navigate the evolving regulatory landscape effectively.
The Role of Documentation in Sterilization Validation
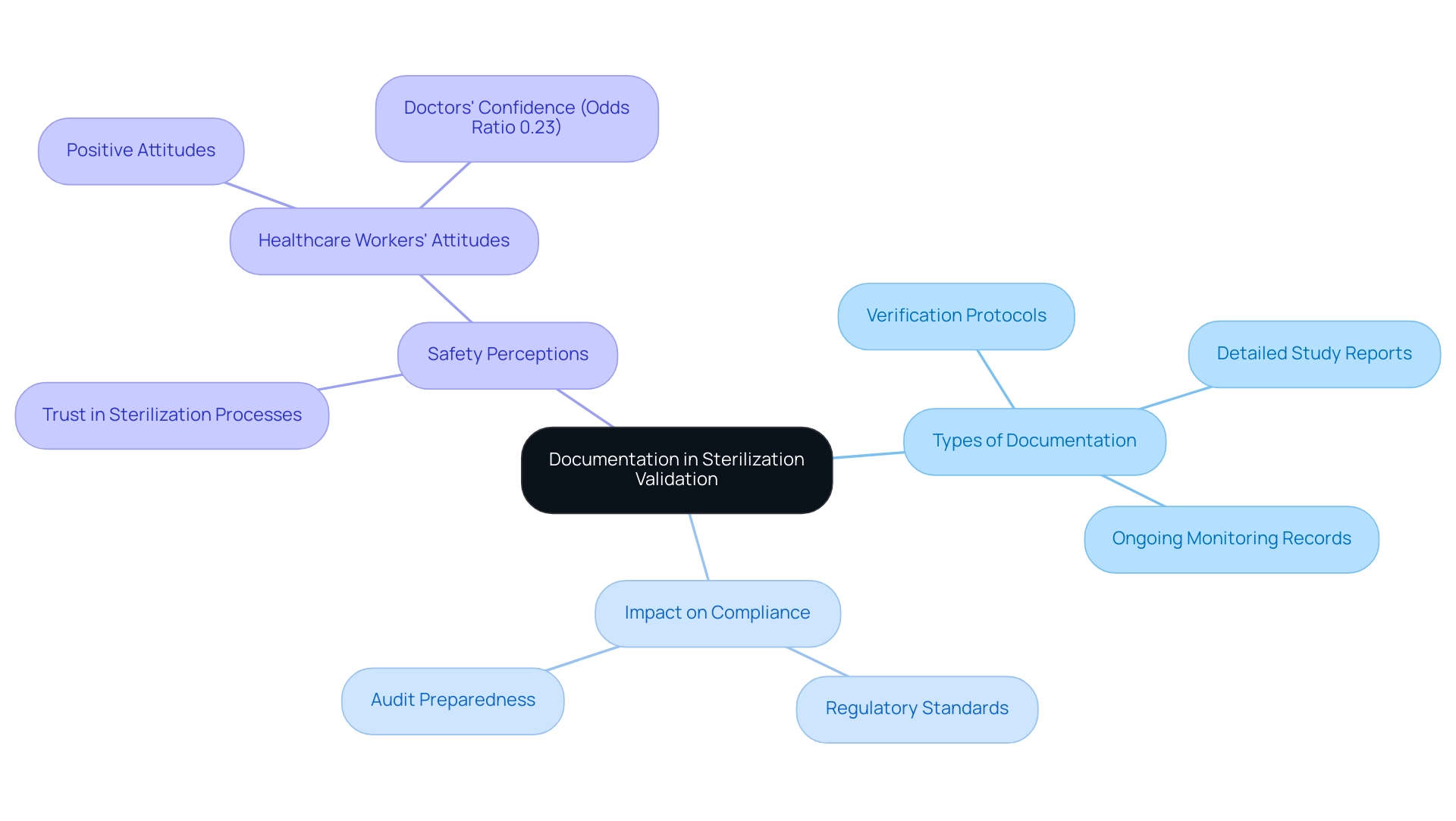
Documentation is essential to the disinfection confirmation procedure, acting as a thorough account of confirmation activities, outcomes, and compliance with regulatory standards. Crucial documents comprise:
- Verification protocols
- Detailed study reports
- Ongoing monitoring records
All of which must be meticulously maintained. Precise and comprehensive documentation not only demonstrates the effectiveness of the sanitization processes but also ensures preparedness for audits by regulatory agencies.
For instance, muslin fabric packs should not exceed dimensions of 12 inches wide × 12 inches high × 20 inches long, with a maximum weight of 12 lbs and a density of 7.2 lbs per cubic foot, serving as a concrete example of documentation standards in disinfection assessment. A robust documentation system promotes transparency and verifiability across all aspects of sterilization validation for medical devices, thereby enhancing the quality assurance. Recent trends indicate that keeping these records is progressively acknowledged as a best practice, with healthcare workers typically exhibiting positive attitudes toward sanitation protocols.
As MD Abdur Rafi, Editor, observes, 'Effective documentation practices are crucial for ensuring compliance and fostering trust in sanitation processes.' Furthermore, a study revealed that while doctors expressed lower confidence in being treated with sterilized devices (Odds Ratio 0.23), the implementation of effective documentation practices can significantly improve perceptions of safety and compliance. As such, it is imperative that clinical research directors prioritize the establishment of effective documentation systems to uphold sterilization validation for medical devices and ensure patient safety.
Conclusion
Achieving sterilization validation is a multifaceted process that is fundamental to the safety and effectiveness of medical devices. From defining the sterilization method to establishing comprehensive protocols, each step plays a crucial role in ensuring that devices are free from harmful pathogens. The emphasis on rigorous testing and detailed documentation cannot be overstated, as these elements are vital for regulatory compliance and patient safety.
Ethylene Oxide sterilization stands out as a critical method, particularly for heat-sensitive items, but it also presents unique challenges that necessitate careful handling and robust validation practices. As the regulatory landscape evolves, adherence to established standards such as ISO 11135 is essential for manufacturers to maintain market access while safeguarding patient health.
Furthermore, the importance of meticulous documentation throughout the sterilization validation process serves not only to verify effectiveness but also to foster transparency and trust in medical practices. By prioritizing these systematic approaches, professionals in the medical device sector can navigate the complexities of sterilization validation, ensuring the highest standards of quality and compliance are met.
In summary, the diligence applied to sterilization validation processes ultimately reflects a commitment to patient safety and operational excellence. As the industry continues to advance, maintaining a focus on these principles will be paramount in delivering safe and effective medical devices to those in need.
Frequently Asked Questions
What is sterilization validation for medical devices?
Sterilization validation for medical devices is a systematic procedure that ensures medical instruments are properly sterilized to eliminate possible pathogens, maintaining patient safety and adhering to regulatory standards.
Why is sterilization validation important?
It is crucial for ensuring patient safety and compliance with stringent regulatory standards, confirming that disinfection methods consistently meet the desired sterility assurance level (SAL).
What does the sterilization validation process involve?
The process involves rigorous testing and thorough documentation to confirm that sterilization methods effectively eliminate pathogens and meet regulatory requirements.
What are the guidelines for documentation related to sterilization validation?
According to FDA guidelines, 510(k) holders must document all qualification activities related to decontamination site changes within their internal files, emphasizing the importance of meticulous record-keeping.
How does sample size affect sterilization qualification?
Insights indicate that the chance of qualification decreases as sample size increases, with specific probabilities of qualification provided for different sample sizes and failure rates, highlighting the role of statistical frameworks in validation.
What is the significance of the EtO Master File Pilot Program?
The EtO Master File Pilot Program for PMA holders illustrates how safety processes influence regulatory procedures, easing submission requirements for Class III medical device producers.
What are the steps involved in the sterilization validation process?
The steps include: 1. Define the sterilization method. 2. Establish verification protocols. 3. Perform pre-validation studies. 4. Execute the verification study. 5. Analyze results. 6. Compile documentation. 7. Implement routine monitoring.
What is the role of statistical analysis in sterilization validation?
Statistical analysis may be necessary to evaluate the success rates of sterilization methods and to assess the effectiveness of the disinfection methods used.
Why is ongoing monitoring important in sterilization validation?
Ongoing monitoring ensures that the disinfection methods remain compliant and effective over time, adapting to any changes in standards or technology to maintain high quality sanitation practices.




